ANTHRASIL Solution for infusion Ref.[9986] Active ingredients: Anthrax immunoglobulin
Source: FDA, National Drug Code (US) Revision Year: 2018
12.1. Mechanism of Action
The polyclonal immune globulin G in ANTHRASIL is a passive immunizing agent that neutralizes anthrax toxin. ANTHRASIL binds to protective antigen (PA) to prevent PA mediated cellular entry of anthrax edema factor and lethal factor. ANTHRASIL is administered in combination with appropriate antibiotic therapy as the product itself is not known to have direct antibacterial activity against anthrax bacteria, which otherwise may continue to grow and product anthrax toxins.
12.3. Pharmacokinetics
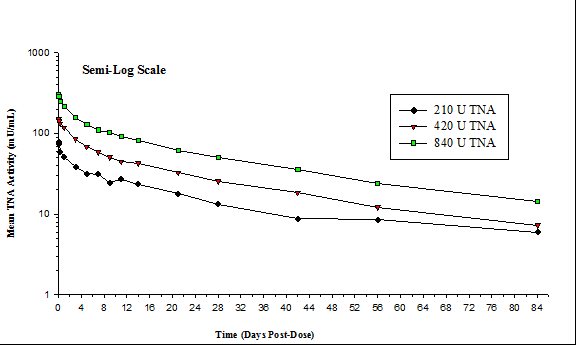
The mean TNA activities for three doses of ANTHRASIL (210, 420 and 840 units TNA) in the clinical trial in healthy volunteers [See 14 CLINICAL STUDIES] are plotted on a semi-log scale in Figure 1. The pharmacokinetics of ANTHRASIL after intravenous infusion of the three dose levels were characterized; the peak levels of ANTHRASIL were reached immediately after infusion and then declined over the duration of study (84 days). The mean TNA activity remained above the lower limit of quantitation (5 milliunits per mL) over the entire 84-day post-dose period for the three doses studied.
Figure 1. Mean TNA Activities for Three Doses of ANTHRASIL:
A summary of the mean pharmacokinetic results for the TNA data collected in the healthy volunteer study is presented in Table 3.
Table 3. Summary of Mean PK Results by Treatment (TNA Data):
| PK Parameters | Dose Levels | |||||
|---|---|---|---|---|---|---|
| 210 U TNA | N | 420 U TNA | N | 840 U TNA | N | |
| Arithmetic Mean (CV%) | ||||||
| AUC0-t (mU·d/mL) | 1031.8 (23.3) | 15 | 2176.7 (18.9) | 17 | 4271.0 (22.3) | 16 |
| AUC0-∞ (mU·d/mL) | 1277.5 (27.7) | 7 | 2536.7 (14.7) | 16 | 4788.8 (26.5) | 15 |
| Cmax (mU/mL) | 83.0 (13.4) | 15 | 156.4 (21.7) | 17 | 316.7 (18.3) | 16 |
| t½ (d) | 24.3 (33.3) | 7 | 28.3 (19.9) | 16 | 28.0 (25.2) | 15 |
| CL (mL/d) | 174.2 (24.1) | 7 | 169.7 (17.9) | 16 | 188.6 (29.5) | 15 |
| Vd (mL) | 5714.8 (11.4) | 7 | 6837.2 (20.4) | 16 | 7238.2 (19.4) | 15 |
| Median (Min-Max) | ||||||
| Tmax (d) | 0.116 (0.109–1.068) | 15 | 0.120 (0.120–0.412) | 17 | 0.169 (0.165–0.459) | 16 |
In comparison to healthy subjects, patients with inhalational anthrax are expected to initially have greater clearance of anti-PA antibodies and lower AUC from ANTHRASIL administration due to the presence of PA antigen.
Mean PK results (TNA data) were evaluated by sex and revealed no sex-related differences over the dose range studied. Systemic exposure of ANTHRASIL increased in a dose-proportional manner over the dose range studied. ANTHRASIL has a serum elimination half-life of 24 to 28 days in healthy humans.
Inhalational anthrax patients, concomitantly treated with antibiotics and a single 420 unit TNA dose of ANTHRASIL, exhibited increases in serum and pleural anti-PA levels; these levels remained at >50% of the peak anti-PA levels over the next five days. The peak serum anti-PA levels in these patients following ANTHRASIL administration (132 to 160 mcg/mL, mean 145 mcg/mL) overlapped with those obtained with the 420 unit dose in healthy volunteers (135 to 250 mcg/mL, mean 190 mcg/mL, median 192 mcg/mL), although mean levels were approximately 25% lower in the inhalational anthrax patients. In the three inhalational anthrax patients, serum and pleural levels of lethal factor declined after initiation of antibiotics and further decreased over the period of five days following ANTHRASIL administration; however, due at least in part to ANTHRASIL targeting the PA component of lethal toxin, plasma and pleural fluid lethal factor levels remained detectable when measured two to five days following ANTHRASIL administration.
Because the effectiveness of ANTHRASIL cannot ethically be tested in placebo-controlled trials in humans, a comparison of ANTHRASIL exposures achieved in healthy human subjects to those observed in animal models of inhalational anthrax in therapeutic efficacy studies was necessary to support the dosage regimen. A dose of 420 units has a similar exposure to the efficacious dose of 15 U/kg administered to New Zealand white rabbits and cynomolgus macaques. In cynomolgus macaques treated with ANTHRASIL monotherapy, a higher dose of 30 U/kg, with a similar exposure to a human dose of 840 units, may result in improved survival [See 13.2 Animal Toxicology and/or Pharmacology]. As a result, the initial dosing regimen is given as a range of 420 to 840 units, and the recommended regimen includes the potential for repeat dosing.
13. Nonclinical Toxicology
Immune globulins are normal constituents of the human body. Toxicology studies have not been performed with ANTHRASIL or its components.
The evaluation of new treatment options for anthrax using placebo-controlled human trials is unethical and infeasible. Therefore, the effectiveness of ANTHRASIL for treatment of inhalational anthrax is based on well controlled efficacy studies conducted in rabbits and cynomolgus macaques.
13.2. Animal Toxicology and/or Pharmacology
Anthrax infected New Zealand white rabbits and cynomolgus macaques administered an intravenous injection of ANTHRASIL (15 units TNA per kg) that did not survive their infection showed an increase in the severity and/or incidence of central nervous system lesions (bacteria, hemorrhage and necrosis) as compared to immune globulin (“placebo”) treated animals who also did not survive the infection. The mean time to death between non-surviving ANTHRASIL and placebo treated animals was comparable. Surviving rabbits had no evidence of central nervous system lesions at the end of the study. No surviving cynomolgus macaques in monotherapeutic studies were tested for central nervous system lesions.
Monotherapeutic Studies in Animal Models
In a monotherapeutic efficacy study, rabbits were exposed to a target dose of 200 x LD50 aerosolized anthrax spores and then administered 15 units per kg of ANTHRASIL at the onset of toxemia, as determined by the presence of PA in serum samples. Detection of PA was used as the trigger for initiation of treatment, while bacteremia status provided a retrospective confirmation of disease. Ninety-eight (98) percent of the treated animals were bacteremic prior to treatment. Of the animals that were toxemic and bacteremic prior to treatment, ANTHRASIL treatment resulted in a 26% survival in comparison to a 2% survival with IGIV placebo treatment (Table 4) over the 36 day duration of the study. ANTHRASIL treatment resulted in a significant decrease in the proportion of rabbits that were toxemic or bacteremic. The time to resolution of toxemia (p=0.0006) or bacteremia (p=0.0074) was also significantly reduced in rabbits that received ANTHRASIL.
Efficacy of ANTHRASIL was also assessed in cynomolgus macaques exposed to a target dose of 200 x LD50 aerosolized anthrax spores. Treatment with placebo or one of three dose levels of ANTHRASIL was initiated after animals became toxemic (positive for PA detection in serum samples), and bacteremia status provided a retrospective confirmation of disease. Survival was assessed over a period of 88 days in toxemic animals that were confirmed to be bacteremic at the time of treatment. Survival was 0% in placebo treated animals. Animals treated with 7.5 units per kg exhibited 36% survival, those treated with 15 units per kg exhibited 43% survival, and those treated with 30 units per kg exhibited 70% survival (Table 4). Compared to placebo, these survival rates were statistically significant at p=0.0451, 0.0339, and 0.0031, respectively. The differences in survival between the 7.5, 15, and 30 unit per kg doses of ANTHRASIL were not statistically significant. ANTHRASIL treated animals showed a statistically significant reduction in anthrax toxin when compared to placebo treated animals.
Table 4 Survival Rates in NZW Rabbits and Cynomolgus Macaques Treated with ANTHRASIL
| NZW Rabbits at 36 Days PI | Cynomolgus Macaques at 28 Days PI | |||
|---|---|---|---|---|
| No. Survivors (%) a | p-Value b | No. Survivors (%) a | p-Value c | |
| Placebo | 1/48 (2) | - | 0/11 (0) | - |
| ANTHRASIL 7.5 U/kg d | - | - | 4/11 (36) | 0.0451 |
| ANTHRASIL 15 U/kg | 13/50 (26) | 0.0009 | 6/14 (43) | 0.0339 |
| ANTHRASIL 30 U/kg d | - | - | 7/10 (70) | 0.0031 |
a Survival among animals that were bacteremic and toxemic prior to treatment
b Two-sided Fisher’s exact test
c Bonferroni-Holm adjusted one-sided Fisher’s exact test
d Dose not evaluated in rabbits in this study
PI = Post-infection
ANTHRASIL Efficacy in Combination with Antibiotics
The efficacy of ANTHRASIL administered with levofloxacin was determined in New Zealand white rabbits with systemic disease. No significant difference between the control (normal immune globulin [IGIV] plus levofloxacin) and treatment groups (ANTHRASIL plus levofloxacin) was seen when combination treatment was delayed up to 60 hours post-challenge. There was no observed antagonism between levofloxacin and ANTHRASIL in this study. This study also supported that ANTHRASIL effectively cleared toxemia when administered with antibiotics. In ANTHRASIL treated groups, all animals cleared PA toxemia post-ANTHRASIL administration and only 4/31 (13%) of ANTHRASIL treated animals exhibited a single transient positive PA result for toxemia at the 12 or 18 hour time point post-dosing. Placebo control animals exhibited more persistent toxemia, with 26/32 (81%) having positive PA results for 18 to 90 hours post-treatment.
In a second study, treatment was delayed beyond 60 hours to simulate a clinical scenario. When combination treatment was initiated at 60, 72, 84 or 96 hours post anthrax exposure, differences in survival were seen, but no statistically significant added survival benefit was observed between groups that received placebo (IGIV plus levofloxacin) or ANTHRASIL (15 units per kg plus levofloxacin). An increase in survival was observed with ANTHRASIL when treatment was delayed to 96 hours post exposure, but was not statistically significant. When treatment was delayed to 96 hours, survival was 25% (2/8) in the antibiotic plus IGIV control group and 71% (5/7) in the ANTHRASIL plus levofloxacin group. A marginal improvement of 10 to 15% was observed at other time points, suggesting a trend in added benefit with ANTHRASIL. This study also demonstrated a significant effect of ANTHRASIL on toxemia. The majority of ANTHRASIL treated animals became negative for PA (toxemia) within one hour post-infusion of ANTHRASIL and remained negative, even with the delayed treatment from 60 to 96 hours post-anthrax challenge and high levels of toxemia pretreatment. In contrast, placebo treated animals remained toxemic up to three days after initiating antibiotic treatment.
The efficacy of ANTHRASIL co-administered with levofloxacin was evaluated in New Zealand white rabbits when treatment was delayed to 96 hours after anthrax spore inhalation. The dose of levofloxacin was chosen to yield a comparable exposure to that achieved by the recommended dose in humans. Of the animals that survived to be treated (19% of those challenged), antibacterial drug plus ANTHRASIL (15 units per kg) resulted in 58% (18/31) survival compared to 39% (13/33) survival in rabbits treated with antibacterial drug and IGIV placebo (p=0.14, Z-test).
When animals were stratified by pre-treatment toxemia (PA) in a post hoc analysis, added benefit was observed in animals treated with ANTHRASIL and levofloxacin when they had pre-treatment PA levels between 200 and 800 ng/mL (p=0.02, Fisher’s exact test). When pre-treatment toxemia was low (PA <200 ng/mL), survival was greater than 90% in all animals, regardless of treatment (Table 5). Animals with very high levels of toxemia (>800 ng/mL) did not survive irrespective of the treatment administered.
Table 5. Survival Rates in New Zealand White Rabbits Stratified by Pre-treatment PA Levels:
| Pre-treatment PA (ng/mL) | IGIV Placebo + Levofloxacin (%) | ANTHRASIL + Levofloxacin (%) |
|---|---|---|
| <200 | 11/12 (91.7) | 8/9 (88.9) |
| 200–800 | 2/11 (18.2) | 10/14 (71.4) |
| >800 | 0/10 (0) | 0/8 (0) |
| All pre-treatment PA levels | 13/33 (39.4) | 18/31 (58.1) |
ANTHRASIL and antibiotic combination treatment was also studied in the cynomolgus macaque model of inhalational anthrax. In this study, delay of initiation of treatment to 64 hours post anthrax exposure resulted in 75% (9/12) survival in the placebo plus ciprofloxacin treatment group versus 83% (10/12) survival in the ANTHRASIL (15 units per kg) plus ciprofloxacin group (p=1).
No antagonism of ANTHRASIL when administered with antibiotic as a concomitant therapy was observed.
ANTHRASIL in Post-exposure Prophylaxis
A post exposure prophylactic study assessed the survival following aerosol exposure to a lethal dose of anthrax spores (200 x LD50) in New Zealand white rabbits administered ANTHRASIL (7.5, 15 or 30 units TNA per kg) at 30 hours post-anthrax challenge compared to placebo controls. All three doses of ANTHRASIL improved survival when given 30 hours post-anthrax challenge. When animals that were both bacteremic and toxemic were treated at 30 hours following challenge, there was a 22% (2/9) survival with a dose of 15 units TNA per kg and a 33% (4/12) survival with a dose of 30 units TNA per kg. All rabbits in the placebo arm died.
14. Clinical Studies
Because it is not ethical or feasible to conduct placebo-controlled clinical trials in humans with inhalational anthrax, the effectiveness of ANTHRASIL is based on efficacy studies demonstrating a survival benefit in animal models of inhalational anthrax infection [See 13.2 Animal Toxicology and/or Pharmacology]. The safety has been assessed in healthy adults and in a limited number of patients with anthrax who were treated with ANTHRASIL under expanded access use.
Safety and Pharmacokinetics of ANTHRASIL in Healthy Volunteers
In a double blind, randomized, placebo-controlled study designed to assess the safety and pharmacokinetics of three doses of ANTHRASIL after a single intravenous infusion in healthy volunteers, a total of 72 healthy adult subjects were randomized to receive a dose of 210, 420 or 840 units of ANTHRASIL by TNA (N=18/dosing group) or an equal volume of saline placebo (N=6/dosing group).
A second stage of this study, designed only for additional safety assessment, was a randomized, open-label study in 20 healthy adult volunteers. Subjects were randomized to receive a dose of 840 units by TNA from one of two additional product lots (10 subjects per lot). There was no placebo group [See 6 ADVERSE REACTIONS and 12.3 Pharmacokinetics].
Patient Experience
Nineteen adult patients have been treated with ANTHRASIL under expanded access use, including three patients with inhalational anthrax, one patient with gastrointestinal anthrax and 15 patients with injectional anthrax due to injection of anthrax-contaminated heroin. Patients were receiving antimicrobial therapy before, during and after ANTHRASIL administration.
In patients with inhalational anthrax, two out of three patients treated with ANTHRASIL plus antimicrobial therapy survived and one died from progression of anthrax disease, systemic candidiasis and multiorgan failure. Among the 15 patients with injectional anthrax treated with ANTHRASIL plus antibiotics, 10 survived and five died (two from progression of anthrax disease; the cause of death was not determined or available for three patients). The single patient with gastrointestinal anthrax treated with ANTHRASIL survived. Therapy for these systemic anthrax cases included aggressive supportive measures including mechanical ventilation and pulmonary/abdominal fluid drainage.
In the three inhalational patients, the ANTHRASIL dose of 420 units by TNA resulted in increased anti-PA levels (correlating with increased TNA activity); these levels remained stable up to seven to 20 days post-administration, probably reflecting the rising antibody production by the patient at the same time that the exogenously-administered antibody was being cleared.
In some injectional anthrax cases, complicated by hemorrhage and pleural and/or peritoneal fluid losses from thoracentesis and/or paracentesis, serum anti-PA antibody levels fell as much as approximately 90% from their post-ANTHRASIL peak levels by 24 hours following ANTHRASIL administration. In the gastrointestinal anthrax patient, serum anti-PA levels were observed prior to ANTHRASIL infusion with further increases in anti-PA levels post-administration and maintenance of anti-PA above pre-administration levels for 11 days was observed.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.