APEXXNAR Suspension for injection Ref.[49639] Active ingredients: Pneumococcus, purified polysaccharides antigen
Source: European Medicines Agency (EU) Revision Year: 2022 Publisher: Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050 Bruxelles, Belgium
4.1. Therapeutic indications
Active immunisation for the prevention of invasive disease and pneumonia caused by Streptococcus pneumoniae in individuals 18 years of age and older.
See sections 4.4 and 5.1 for information on protection against specific pneumococcal serotypes.
Apexxnar should be used in accordance with official recommendations.
4.2. Posology and method of administration
Posology
Individuals 18 years of age and older
Apexxnar is to be administered as a single dose to individuals 18 years of age and older.
The need for revaccination with a subsequent dose of Apexxnar has not been established.
No data on sequential vaccination with other pneumococcal vaccines or a booster dose are available for Apexxnar. Based on the clinical experience with Prevenar 13 (a pneumococcal conjugate vaccine consisting of 13 polysaccharide conjugates that are also in Apexxnar), if the use of 23-valent pneumococcal polysaccharide vaccine (Pneumovax 23 [PPSV23]) is considered appropriate, Apexxnar should be given first (see section 5.1).
Paediatric population
The safety and efficacy of Apexxnar in children and adolescents younger than 18 years of age have not been established. No data are available.
Special populations
There are no data with Apexxnar in special populations.
Limited experience from clinical studies with Prevenar 13 (a pneumococcal conjugate vaccine consisting of 13 polysaccharide conjugates that are also in Apexxnar) are available in adults at higher risk of pneumococcal infection either immunocompromised individuals or following bone marrow transplantation (see sections 4.4 and 5.1).
Based on these data the following posology was recommended for Prevenar 13:
- Individuals at higher risk of pneumococcal infection (e.g., individuals with sickle cell disease or HIV infection), including those previously vaccinated with 1 or more doses of PPSV23, were recommended to receive at least 1 dose of Prevenar 13.
- In individuals with a hematopoietic stem cell transplant (HSCT), the recommended immunisation series with Prevenar 13 consisted of 4 doses of 0.5 mL each. The primary series consisted of 3 doses, with the first dose given 3 to 6 months after HSCT and with an interval of at least 1 month between doses. A booster dose was recommended 6 months after the third dose (see section 5.1).
Please also refer to sections 4.4. and 5.1.
Method of administration
For intramuscular use only.
One dose (0.5 mL) of Apexxnar should be administered intramuscularly, preferably in the deltoid muscle, with care to avoid injection into or near nerves and blood vessels.
For instructions on the handling of the vaccine before administration, see section 6.6.
4.9. Overdose
Overdose with Apexxnar is unlikely due to its presentation as a pre-filled syringe.
6.3. Shelf life
24 months.
6.4. Special precautions for storage
Store in a refrigerator (2°C to 8°C). Pre-filled syringes should be stored in the refrigerator horizontally to minimise the resuspension time.
Do not freeze. Discard if the vaccine has been frozen.
From a microbiological point of view, once removed from the refrigerator, the vaccine should be used immediately.
Stability data indicate that the vaccine is stable for 96 hours when stored at temperatures from 8°C to 25°C, or 72 hours when stored at temperatures from 0°C to 2°C. At the end of these time periods Apexxnar should be used or discarded. These data are intended to guide healthcare professionals in case of temporary temperature excursion only.
6.5. Nature and contents of container
0.5 mL suspension for injection in pre-filled syringe (Type I glass) with a tip cap (synthetic isoprene/bromobutyl blend rubber) and a plunger stopper (chlorobutyl rubber).
Pack sizes of 1, 10, and multipack of 50 (5 × 10) prefilled syringes, with or without needle.
Not all pack sizes may be marketed.
6.6. Special precautions for disposal and other handling
During storage, a white deposit and clear supernatant may be observed in the pre-filled syringe containing the suspension. Pre-filled syringes should be stored horizontally to minimise the resuspension time.
Preparation for administration
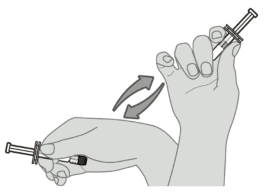
Step 1. Vaccine resuspension
Hold the pre-filled syringe horizontally between the thumb and the forefinger and shake vigorously until the contents of the syringe are a homogeneous white suspension. Do not use the vaccine if it cannot be resuspended.
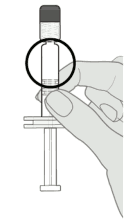
Step 2. Visual inspection
Visually inspect the vaccine for large particulate matter and discolouration prior to administration. Do not use if large particulate matter or discolouration is found. If the vaccine is not a homogenous white suspension, repeat steps 1 and 2.
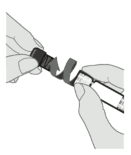
Step 3. Remove syringe cap
Remove the syringe cap from the Luer lock adapter by slowly turning the cap counter clockwise while holding the Luer lock adapter.
Note: Care should be taken to ensure that the extended plunger rod is not depressed while removing the syringe cap.
Step 4. Attach a sterile needle
Attach a needle appropriate for intramuscular administration to the pre-filled syringe by holding the Luer lock adapter and turning the needle clockwise.
Any unused product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.