APIDRA Solution for injection Ref.[9168] Active ingredients: Insulin glulisine
Source: European Medicines Agency (EU) Revision Year: 2020 Publisher: Sanofi-Aventis Deutschland GmbH, D-65926, Frankfurt am Main, Germany
Therapeutic indications
Treatment of adults, adolescents and children 6 years or older, with diabetes mellitus, where treatment with insulin is required.
Posology and method of administration
Posology
The potency of this preparation is stated in units. These units are exclusive to Apidra and are not the same as IU or the units used to express the potency of other insulin analogues (see section 5.1). Apidra should be used in regimens that include an intermediate or long acting insulin or basal insulin analogue and can be used with oral hypoglycaemic agents. The dose of Apidra should be individually adjusted.
Special populations
Renal impairment
The pharmacokinetic properties of insulin glulisine are generally maintained in patients with renal impairment. However, insulin requirements may be reduced in the presence of renal impairment (see section 5.2).
Hepatic impairment
The pharmacokinetic properties of insulin glulisine have not been investigated in patients with decreased liver function. In patients with hepatic impairment, insulin requirements may be diminished due to reduced capacity for gluconeogenesis and reduced insulin metabolism.
Elderly
Limited pharmacokinetic data are available in elderly patients with diabetes mellitus. Deterioration of renal function may lead to a decrease in insulin requirements.
Paediatric population
There is insufficient clinical information on the use of Apidra in children younger than the age of 6 years.
Method of administration
Apidra 100 Units/ml solution for injection in a vial
Intravenous use
Apidra can be administered intravenously. This should be carried out by healthcare professionals. Apidra must not be mixed with glucose or Ringer’s solution or with any other insulin.
Continuous subcutaneous insulin infusion
Apidra may be used for Continuous Subcutaneous Insulin Infusion (CSII) in pump systems suitable for insulin infusion with the appropriate catheters and reservoirs. Patients using CSII should be comprehensively instructed on the use of the pump system.
The infusion set and reservoir used with Apidra must be changed at least every 48 hours using aseptic technique. These instructions may differ from general pump manual instructions. It is important that patients follow the Apidra specific instructions when using Apidra. Failure to follow Apidra specific instructions may lead to serious adverse events.
When used with a subcutaneous insulin infusion pump, Apidra must not be mixed with diluents or any other insulin.
Patients administering Apidra by CSII must have an alternative insulin delivery system available in case of pump system failure (see section 4.4 and 4.8).
Apidra 100 Units/ml solution for injection in a vial
For further details on handling, see section 6.6.
Apidra 100 Units/ml solution for injection in a cartridge
Apidra 100 Units/ml in cartridges is only suitable for subcutaneous injections from a reusable pen. If administration by syringe, intravenous injection or infusion pump is necessary, a vial should be used (see section 4.4). For further details on handling, see section 6.6.
Apidra SoloStar 100 Units/ml solution for injection in a pre-filled pen
Apidra SoloStar 100 Units/ml in pre-filled pen is only suitable for subcutaneous injections. If administration by syringe, intravenous injection or infusion pump is necessary, a vial should be used (see section 4.4).
Subcutaneous use
Apidra should be given by subcutaneous injection shortly (0-15 min) before or soon after meals or by continuous subcutaneous pump infusion.
Apidra should be administered subcutaneously in the abdominal wall, thigh or deltoid or by continuous infusion in the abdominal wall. Injection sites and infusion sites within an injection area (abdomen, thigh or deltoid) should be rotated from one injection to the next. The rate of absorption, and consequently the onset and duration of action, may be affected by the injection site, exercise and other variables.
Subcutaneous injection in the abdominal wall ensures a slightly faster absorption than other injection sites (see section 5.2).
Care should be taken to ensure that a blood vessel has not been entered. After injection, the site of injection should not be massaged. Patients must be educated to use proper injection techniques.
Mixing with insulins
When administered as a subcutaneous injection, Apidra must not be mixed with other medicinal products except NPH human insulin.
For further details on handling, see section 6.6.
Before using SoloStar, the Instructions for use included in the Package leaflet must be read carefully (see section 6.6).
Overdose
Symptoms
Hypoglycaemia may occur as a result of an excess of insulin activity relative to food intake and energy expenditure.
There are no specific data available concerning overdoses with insulin glulisine. However, hypoglycaemia may develop over sequential stages.
Management
Mild hypoglycaemic episodes can be treated by oral administration of glucose or sugary products. It is therefore recommended that the diabetic patient constantly carries some sugar lumps, sweets, biscuits or sugary fruit juice.
Severe hypoglycaemic episodes, where the patient has become unconscious, can be treated by glucagon (0.5 mg to 1 mg) given intramuscularly or subcutaneously by a person who has received appropriate instruction, or by glucose given intravenously by a healthcare professional. Glucose must also be given intravenously, if the patient does not respond to glucagon within 10 to 15 minutes.
Upon regaining consciousness, administration of oral carbohydrate is recommended for the patient in order to prevent relapse.
After an injection of glucagon, the patient should be monitored in a hospital in order to find the reason for this severe hypoglycaemia and prevent other similar episodes.
Shelf life
Shelf life: 2 years.
Apidra 100 Units/ml solution for injection in a vial
Shelf life after first use of the vial: The product may be stored for a maximum of 4 weeks below 25°C away from direct heat or direct light. Keep the vial in the outer carton in order to protect from light. It is recommended that the date of the first use from the vial be noted on the label.
Shelf life for intravenous use: Insulin glulisine for intravenous use at a concentration of 1 Unit/ml is stable between 15°C and 25°C for 48 hours (see section 6.6).
Apidra 100 Units/ml solution for injection in a cartridge
Shelf life after first use of the cartridge: The product may be stored for a maximum of 4 weeks below 25°C away from direct heat or direct light. The pen containing a cartridge must not be stored in the refrigerator. The pen cap must be put back on the pen after each injection in order to protect from light.
Apidra SoloStar 100 Units/ml solution for injection in a pre-filled pen
Shelf life after first use of the pen: The product may be stored for a maximum of 4 weeks below 25°C away from direct heat or direct light. Pens in use must not be stored in the refrigerator. The pen cap must be put back on the pen after each injection in order to protect from light.
Special precautions for storage
Apidra 100 Units/ml solution for injection in a vial
Unopened vials:
Store in a refrigerator (2°C-8°C).
Do not freeze.
Do not put Apidra next to the freezer compartment or a freezer pack.
Keep the vial in the outer carton in order to protect from light.
Opened vials:
For storage conditions after first opening of the medicinal product, see section 6.3.
Apidra 100 Units/ml solution for injection in a cartridge
Unopened cartridges:
Store in a refrigerator (2°C-8°C).
Do not freeze.
Do not put Apidra next to the freezer compartment or a freezer pack.
Keep the cartridge in the outer carton in order to protect from light.
In-use cartridges:
For storage conditions after first opening of the medicinal product, see section 6.3.
Apidra SoloStar 100 Units/ml solution for injection in a pre-filled pen
Not in-use pens:
Store in a refrigerator (2°C-8°C).
Do not freeze.
Do not put Apidra next to the freezer compartment or a freezer pack.
Keep the pre-filled pen in the outer carton in order to protect from light.
In-use pens:
For storage conditions after first opening of the medicinal product, see section 6.3.
Nature and contents of container
Apidra 100 Units/ml solution for injection in a vial: 10 ml solution in a vial (type I colourless glass) with a stopper (flanged aluminium overseal, elastomeric chlorobutyl rubber) and a polypropylene tear-off cap. Packs of 1, 2, 4 and 5 vials are available.
Not all pack sizes may be marketed.
Apidra 100 Units/ml solution for injection in a cartridge: 3 ml solution in a cartridge (type I colourless glass) with a plunger (elastomeric bromobutyl rubber) and a flanged cap (aluminium) with a stopper (elastomeric bromobutyl rubber). Packs of 1, 3, 4, 5, 6, 8, 9 and 10 cartridges are available.
Not all pack sizes may be marketed.
Apidra SoloStar 100 Units/ml solution for injection in a pre-filled pen: 3 ml solution in a cartridge (colourless glass) with a plunger (elastomeric bromobutyl rubber) and a flanged cap (aluminium) with a stopper (elastomeric bromobutyl rubber). The cartridge is sealed in a disposable pre-filled pen. Packs of 1, 3, 4, 5, 6, 8, 9 and 10 pens are available.
Not all pack sizes may be marketed.
Special precautions for disposal and other handling
Apidra 100 Units/ml solution for injection in a vial
Subcutaneous use
Apidra vials are for use with insulin syringes with the corresponding unit scale and for use with an insulin pump system (see section 4.2). Inspect the vial before use. It must only be used if the solution is clear, colourless, with no solid particles visible. Since Apidra is a solution, it does not require resuspension before use. Insulin label must always be checked before each injection to avoid medication errors between insulin glulisine and other insulins (see section 4.4).
Mixing with insulins
When mixed with NPH human insulin, Apidra should be drawn into the syringe first. Injection should be given immediately after mixing as no data are available regarding the mixtures made up a significant time before injection.
Continuous subcutaneous infusion pump
Refer to section 4.2 and 4.4 for advice.
Intravenous use
Apidra should be used at a concentration of 1 Unit/ml insulin glulisine in infusion systems with sodium chloride 9 mg/ml (0.9%) solution for infusion with or without 40 mmol/l potassium chloride using coextruded polyolefin/polyamide plastic infusion bags with a dedicated infusion line. Insulin glulisine for intravenous use at a concentration of 1 Unit/ml is stable at room temperature for 48 hours.
After dilution for intravenous use, the solution should be inspected visually for particulate matter prior to administration. It must only be used if the solution is clear and colourless, not when cloudy or with visible particles.
Apidra was found to be incompatible with Glucose 5% solution and Ringer’s solution and, therefore, must not be used with these solution fluids. The use of other solutions has not been studied.
Apidra 100 Units/ml solution for injection in a cartridge
Apidra 100 units/ml in a cartridge is only suitable for subcutaneous injections from a reusable pen. If administration by syringe, intravenous injection or infusion pump is necessary, a vial should be used. The Apidra cartridges are to be used only in conjunction with the pens: ClikSTAR, Autopen 24, Tactipen, AllStar, AllStar PRO or JuniorSTAR (see section 4.2 and 4.4). Not all of these pens may be marketed in your country.
The pen should be used as recommended in the information provided by the device manufacturer.
The manufacturer’s instructions for using the pen must be followed carefully for loading the cartridge, attaching the needle, and administering the insulin injection. Inspect the cartridge before use. It must only be used if the solution is clear, colourless, with no solid particles visible. Before insertion of the cartridge into the reusable pen, the cartridge must be stored at room temperature for 1 to 2 hours. Air bubbles must be removed from the cartridge before injection (see instruction for using pen). Empty cartridges must not be refilled.
If the insulin pen is damaged or not working properly (due to mechanical defects) it has to be discarded, and a new insulin pen has to be used.
To prevent any kind of contamination, the re-usable pen should be used by a single patient only. Insulin label must always be checked before each injection to avoid medication errors between insulin glulisine and other insulins (see section 4.4).
Apidra SoloStar 100 Units/ml solution for injection in a pre-filled pen
Apidra SoloStar 100 units/ml in a pre-filled pen is only suitable for subcutaneous injections. If administration by syringe, intravenous injection or infusion pump is necessary, a vial should be used.
Before first use, the pen must be stored at room temperature for 1 to 2 hours.
Inspect the cartridge before use. It must only be used if the solution is clear, colourless, with no solid particles visible, and if it is of water-like consistency. Since Apidra is a solution, it does not require resuspension before use.
Empty pens must never be reused and must be properly discarded.
To prevent any kind of contamination, the use of the pre-filled pen should remain strictly for a single patient use.
Insulin label must always be checked before each injection to avoid medication errors between insulin glulisine and other insulins (see section 4.4).
Handling of the pen
The patient should be advised to read the instructions for use included in the package leaflet carefully before using SoloStar.
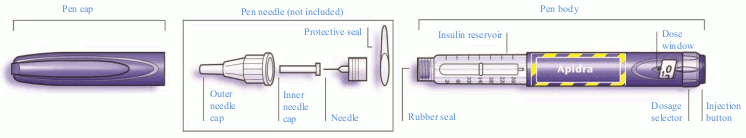
Schematic diagram of the pen:
Important information for use of SoloStar:
- Before each use, a new needle must always be carefully attached and a safety test must be performed. A dose should not be selected and/or the injection button should not be pressed without a needle attached. Only use needles that are compatible for use with SoloStar.
- Special caution must be taken to avoid accidental needle injury and transmission of infection.
- SoloStar must never be used if it is damaged or if the patient is not sure if it is working properly.
- The patient must always have a spare SoloStar available in case the SoloStar is lost or damaged.
Storage instructions:
Please check section 6.4 of this SPC for instructions on how to store SoloStar.
If SoloStar is in cool storage, it should be taken out 1 to 2 hours before you inject to allow it to warm up. Cold insulin is more painful to inject.
The used SoloStar must be discarded as required by your local authorities.
Maintenance:
SoloStar has to be protected from dust and dirt.
The outside of the SoloStar can be cleaned by wiping it with a damp cloth.
The pen must not be soaked, washed or lubricated as this may damage it.
SoloStar is designed to work accurately and safely. It should be handled with care. The patient should avoid situations where SoloStar may be damaged. If the patient is concerned that the SoloStar may be damaged, he must use a new one.
Step 1 Check the insulin
The label on the pen should be checked to make sure it contains the correct insulin. The Apidra SoloStar is blue. It has a dark blue injection button with a raised ring on the top. After removing the pen cap, the appearance of insulin should also be checked: the insulin solution must be clear, colourless, with no solid particles visible, and must have a water-like consistency.
Step 2 Attach the needle
Only needles that are compatible for use with SoloStar should be used. A new sterile needle will be always used for each injection. After removing the cap, the needle should be carefully attached straight onto the pen.
Step 3 Perform a safety test
Prior to each injection a safety test has to be performed to ensure that pen and needle work properly and to remove air bubbles.
A dose of 2 units has to be selected.
The outer and inner needle caps should be removed.
While holding the pen with the needle pointing upwards, the insulin reservoir should be tapped gently with the finger so that any air bubbles rise up towards the needle.
Then the injection button should be pressed in completely.
If insulin has been expelled through the needle tip, then the pen and the needle are working properly.
If no insulin appears at the needle tip, step 3 should be repeated until insulin appears at the needle tip.
Step 4 Select the dose
The dose can be set in steps of 1 unit, from a minimum of 1 unit to a maximum of 80 units. If a dose greater than 80 units is required, it should be given as two or more injections.
The dose window must show “0” following the safety test. The dose can then be selected.
Step 5 Inject the dose
The patient should be informed on the injection technique by his health care professional.
The needle should be inserted into the skin.
The injection button should be pressed in completely. Then the injection button should be held down 10 seconds before withdrawing the needle. This ensures that the full dose of insulin has been injected.
Step 6 Remove and discard the needle
The needle should always be removed after each injection and discarded. This helps prevent contamination and/or infection, entry of air into the insulin reservoir and leakage of insulin. Needles must not be reused.
Special caution must be taken when removing and disposing the needle. Recommended safety measures for removal and disposal of needles must be followed (e.g. a one handed capping technique) in order to reduce the risk of accidental needle injury and transmission of infectious diseases.
The pen cap should be replaced on the pen.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.