ARESTIN Powder for solution for injection Ref.[10750] Active ingredients: Minocycline
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
ARESTIN (minocycline hydrochloride) microspheres, 1 mg is a subgingival sustained-release product containing the antibiotic minocycline hydrochloride incorporated into a bioresorbable polymer, Poly (glycolide-co-dl-lactide) or PGLA, for professional subgingival administration into periodontal pockets. Each unit-dose cartridge delivers minocycline hydrochloride equivalent to 1 mg of minocycline free base.
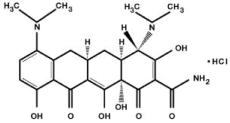
The molecular formula of minocycline hydrochloride is C23H27N3O7·HCl, and the molecular weight is 493.94.
The structural formula of minocycline hydrochloride is:
| How Supplied |
|---|
|
ARESTIN (minocycline hydrochloride) microspheres, 1 mg is supplied as follows: NDC 65976-100-01 1 unit-dose cartridge with desiccant in a heat-sealed, foil-laminated pouch NDC 65976-100-24 12 unit-dose cartridges in 1 tray with desiccant in a heat-sealed, foil-laminated, resealable pouch. There are 2 pouches in each box. Each unit-dose cartridge contains the product identifier “OP-1”. Distributed by: OraPharma, a division of Bausch Health US, LLC, Bridgewater, NJ 08807 USA |
Drugs
| Drug | Countries | |
|---|---|---|
| ARESTIN | Canada, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.