ARMISARTE Concentrate for solution for infusion Ref.[8457] Active ingredients: Pemetrexed
Source: European Medicines Agency (EU) Revision Year: 2019 Publisher: Actavis Group PTC ehf., Reykjavíkurvegi 76-78, 220 Hafnarfjörður, Iceland
Pharmacodynamic properties
Pharmacotherapeutic group: antineoplastic agents, folic acid analogues
ATC code: L01BA04
Pemetrexed is a multi-targeted anti-cancer antifolate agent that exerts its action by disrupting crucial folate-dependent metabolic processes essential for cell replication.
In vitro studies have shown that pemetrexed behaves as a multitargeted antifolate by inhibiting thymidylate synthase (TS), dihydrofolate reductase (DHFR), and glycinamide ribonucleotide formyltransferase (GARFT), which are key folate-dependent enzymes for the de novo biosynthesis of thymidine and purine nucleotides. Pemetrexed is transported into cells by both the reduced folate carrier and membrane folate binding protein transport systems. Once in the cell, pemetrexed is rapidly and efficiently converted to polyglutamate forms by the enzyme folylpolyglutamate synthetase. The polyglutamate forms are retained in cells and are even more potent inhibitors of TS and GARFT. Polyglutamation is a time- and concentration-dependent process that occurs in tumour cells and, to a lesser extent, in normal tissues. Polyglutamated metabolites have an increased intracellular half-life resulting in prolonged drug action in malignant cells.
The European Medicines Agency has waived the obligation to submit the results of studies with pemetrexed in all subsets of the paediatric population in the granted indications (see section 4.2).
Clinical efficacy
Mesothelioma
EMPHACIS, a multicentre, randomised, single-blind phase 3 study of pemetrexed plus cisplatin versus cisplatin in chemonaive patients with malignant pleural mesothelioma, has shown that patients treated with pemetrexed and cisplatin had a clinically meaningful 2.8-month median survival advantage over patients receiving cisplatin alone.
During the study, low-dose folic acid and vitamin B12 supplementation was introduced to patients therapy to reduce toxicity. The primary analysis of this study was performed on the population of all patients randomly assigned to a treatment arm who received study drug (randomised and treated). A subgroup analysis was performed on patients who received folic acid and vitamin B12 supplementation during the entire course of study therapy (fully supplemented). The results of these analyses of efficacy are summarised in the table below:
Efficacy of pemetrexed plus cisplatin vs. cisplatin in malignant pleural mesothelioma:
| Randomized and treated patients | Fully supplemented Patients | |||
|---|---|---|---|---|
| Efficacy parameter | Pemetrexed/cisplatin (Ν=226) | Cisplatin (Ν=222) | Pemetrexed/cisplatin (Ν=168) | Cisplatin (Ν=163) |
| Median overall survival (months) (95% CI) | 12.1 (10.0–14.4) | 9.3 (7.8–10.7) | 13.3 (11.4–14.9) | 10.0 (8.4–11.9) |
| Log Rank p-value* | 0.020 | 0.051 | ||
| Median time to tumour progression (months) (95% CI) | 5.7 (4.9–6.5) | 3.9 (2.8–4.4) | 6.1 (5.3–7.0) | 3.9 (2.8–4.5) |
| Log Rank p-value* | 0.001 | 0.008 | ||
| Time to treatment failure (months) (95% CI) | 4.5 (3.9–4.9) | 2.7 (2.1–2.9) | 4.7 (4.3–5.6) | 2.7 (2.2–3.1) |
| Log Rank p-value* | 0.001 | 0.001 | ||
| Overall response rate** (95% CI) | 41.3% (34.8–48.1) | 16.7% (12.0–22.2) | 45.5% (37.8–53.4) | 19.6% (13.8–26.6) |
| Fisher's exact p-value* | <0,001 | <0,001 | ||
Abbreviation: CI = confidence interval
* p-value refers to comparison between arms.
** In the pemetrexed/cisplatin arm, randomized and treated (N=225) and fully supplemented (N=167)
A statistically significant improvement of the clinically relevant symptoms (pain and dyspnoea) associated with malignant pleural mesothelioma in the pemetrexed/cisplatin arm (212 patients) versus the cisplatin arm alone (218 patients) was demonstrated using the Lung Cancer Symptom Scale. Statistically significant differences in pulmonary function tests were also observed. The separation between the treatment arms was achieved by improvement in lung function in the pemetrexed/cisplatin arm and deterioration of lung function over time in the control arm.
There are limited data in patients with malignant pleural mesothelioma treated with pemetrexed alone. Pemetrexed at a dose of 500 mg/m² was studied as a single-agent in 64 chemonaive patients with malignant pleural mesothelioma. The overall response rate was 14.1%.
NSCLC, second-line treatment
A multicentre, randomised, open label phase 3 study of pemetrexed versus docetaxel in patients with locally advanced or metastatic NSCLC after prior chemotherapy has shown median survival times of 8.3 months for patients treated with pemetrexed (Intent To Treat population n=283) and 7.9 months for patients treated with docetaxel (ITT n=288). Prior chemotherapy did not include pemetrexed. An analysis of the impact of NSCLC histology on the treatment effect on overall survival was in favour of pemetrexed versus docetaxel for other than predominantly squamous histologies (n=399, 9.3 versus 8.0 months, adjusted HR=0.78; 95% CI=0.61-1.00, p=0.047) and was in favour of docetaxel for squamous cell carcinoma histology (n=172, 6.2 versus 7.4 months, adjusted HR=1.56; 95% CI=1.08-2.26, p=0.018). There were no clinically relevant differences observed for the safety profile of pemetrexed within the histology subgroups.
Limited clinical data from a separate randomized, Phase 3, controlled trial, suggest that efficacy data (overall survival, progression free survival) for pemetrexed are similar between patients previously pre-treated with docetaxel (n=41) and patients who did not receive previous docetaxel treatment (n=540).
Efficacy of pemetrexed vs docetaxel in NSCLC - ITT population:
| Pemetrexed | Docetaxel | |
|---|---|---|
| Survival Time (months) | (n=283) | (n=288) |
| Median (m) | 8.3 | 7.9 |
| 95% CI for median | (7.0–9.4) | (6.3–9.2) |
| HR | 0.99 | |
| 95% CI for HR | (0.82–1.20) | |
| Non-inferiority p-value (HR) | 0.226 | |
| Progression free survival (months) | (n=283) | (n=288) |
| Median | 2.9 | 2.9 |
| HR (95% CI) | 0.97 (0.82–1.16) | |
| Χρόνος αποτυχίας της θεραπείας (μήνες) | (n=283) | =288) |
| Median | 2.3 | 2.1 |
| HR (95% CI) | 0.84 (0.71–0.997) | |
| Response (n: qualified for response) | (n=264) | (n=274) |
| Response rate (%) (95% CI) | 9.1 (5.9–13.2) | 8.8 (5.7–12.8) |
| Stable disease (%) | 45.8 | 46.4 |
Abbreviations: CI = confidence interval; HR = hazard ratio; ITT = intent to treat; n = total population size.
NSCLC, first-line treatment
A multicentre, randomised, open-label, Phase 3 study of pemetrexed plus cisplatin versus gemcitabine plus cisplatin in chemonaive patients with locally advanced or metastatic (Stage IIIb or IV) non-small cell lung cancer (NSCLC) showed that pemetrexed plus cisplatin (Intent-To-Treat [ITT] population n=862) met its primary endpoint and showed similar clinical efficacy as gemcitabine plus cisplatin (ITT n=863) in overall survival (adjusted hazard ratio 0.94; 95% CI=0.84-1.05). All patients included in this study had an ECOG performance status 0 or 1.
The primary efficacy analysis was based on the ITT population. Sensitivity analyses of main efficacy endpoints were also assessed on the Protocol Qualified (PQ) population. The efficacy analyses using PQ population are consistent with the analyses for the ITT population and support the non-inferiority of AC versus GC.
Progression free survival (PFS) and overall response rate were similar between treatment arms: median PFS was 4.8 months for pemetrexed plus cisplatin versus 5.1 months for gemcitabine plus cisplatin (adjusted hazard ratio 1.04; 95% CI=0.94-1.15), and overall response rate was 30.6% (95% CI=27.3-33.9) for pemetrexed plus cisplatin versus 28.2% (95% CI=25.0-31.4) for gemcitabine plus cisplatin. PFS data were partially confirmed by an independent review (400/1725 patients were randomly selected for review). The analysis of the impact of NSCLC histology on overall survival demonstrated clinically relevant differences in survival according to histology, see table below.
Efficacy of pemetrexed + cisplatin vs. gemcitabine + cisplatin in first-line non-small cell lung cancer – ITT population and histology subgroups:
| ITT population and histology subgroups | Median overall survival in months (95% CI) | Adjusted hazard ratio (HR) (95% CI) | Superiority p-value | |||
|---|---|---|---|---|---|---|
| Pemetrexed + cisplatin | Gemcitabine + cisplatin | |||||
| ITT population (N=1725) | 10.3 (9.8–11.2) | N=862 | 10.3 (9.6–10.9) | N=863 | 0.94a (0.84–1.05) | 0.259 |
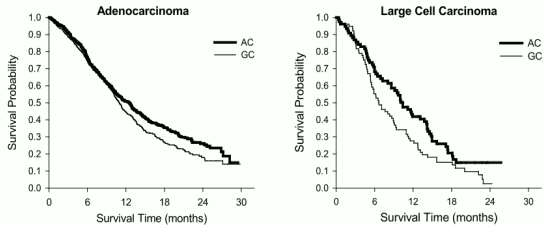
| Adenocarcinoma (N=847) | 12.6 (10.7-13.6) | N=436 | 10.9 (10.2–11.9) | N=411 | 0.84 (0.71–0.99) | 0.033 |
| Large cell (N=153) | 10.4 (8.6–14.1) | N=76 | 6.7 (5.5–9.0) | N=77 | 0.67 (0.48–0.96) | 0.027 |
| Other (N=252) | 8.6 (6.8–10.2) | N=106 | 9.2 (8.1–10.6) | N=146 | 1.08 (0.81–1.45) | 0.586 |
| Squamous cell (N=473) | 9.4 (8.4–10.2) | N=244 | 10.8 (9.5–12.1) | N=229 | 1.23 (1.00–1.51) | 0.050 |
Abbreviations: CI = confidence interval; ITT = intent-to-treat; N = total population size.
a Statistically significant for noninferiority, with the entire confidence interval for HR well below the 1.17645 noninferiority margin (p<0.001).
Kaplan Meier plots of overall survival by histology:
There were no clinically relevant differences observed for the safety profile of pemetrexed plus cisplatin within the histology subgroups. Patients treated with pemetrexed and cisplatin required fewer transfusions (16.4% versus 28.9%, p<0.001), red blood cell transfusions (16.1% versus 27.3%, p<0.001) and platelet transfusions (1.8% versus 4.5%, p=0.002). Patients also required lower administration of erythropoietin/darbopoietin (10.4% versus 18.1%, p<0.001), G-CSF/GM-CSF (3.1% versus 6.1%, p=0.004), and iron preparations (4.3% versus 7.0%, p=0.021).
NSCLC, maintenance treatment
JMEN
A multicentre, randomised, double-blind, placebo-controlled Phase 3 study (JMEN), compared the efficacy and safety of maintenance treatment with pemetrexed plus best supportive care (BSC) (n=441) with that of placebo plus BSC (n=222) in patients with locally advanced (Stage IIIB) or metastatic (Stage IV) Non Small Cell Lung Cancer (NSCLC) who did not progress after 4 cycles of first line doublet therapy containing Cisplatin or Carboplatin in combination with Gemcitabine, Paclitaxel, or Docetaxel. First line doublet therapy containing pemetrexed was not included. All patients included in this study had an ECOG performance status 0 or 1. Patients received maintenance treatment until disease progression. Efficacy and safety were measured from the time of randomisation after completion of first line (induction) therapy. Patients received a median of 5 cycles of maintenance treatment with pemetrexed and 3.5 cycles of placebo. A total of 213 patients (48.3%) completed ≥6 cycles and a total of 103 patients (23.4%) completed ≥10 cycles of treatment with pemetrexed.
The study met its primary endpoint and showed a statistically significant improvement in PFS in the pemetrexed arm over the placebo arm (n=581, independently reviewed population; median of 4.0 months and 2.0 months, respectively) (hazard ratio=0.60, 95% CI=0.49-0.73, p<0.00001). The independent review of patient scans confirmed the findings of the investigator assessment of PFS. The median OS for the overall population (n=663) was 13.4 months for the pemetrexed arm and 10.6 months for the placebo arm, hazard ratio=0.79 (95% CI=0.65-0.95, p=0.01192).
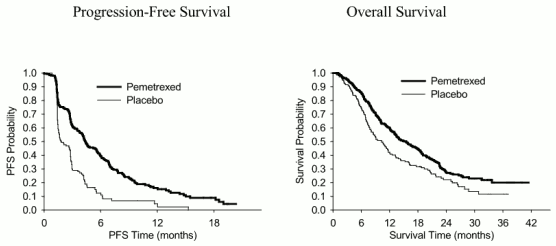
Consistent with other pemetrexed studies, a difference in efficacy according to NSCLC histology was observed in JMEN. For patients with NSCLC other than predominantly squamous cell histology (n=430, independently reviewed population) median PFS was 4.4 months for the pemetrexed arm and 1.8 months for the placebo arm, hazard ratio=0.47 (95% CI=0.37-0.60, p=0.00001). The median OS for patients with NSCLC other than predominantly squamous cell histology (n=481) was 15.5 months for the pemetrexed arm and 10.3 months for the placebo arm, hazard ratio=0.70 (95% CI=0.56-0.88, p=0.002). Including the induction phase the median OS for patients with NSCLC other than predominantly squamous cell histology was 18.6 months for the pemetrexed arm and 13.6 months for the placebo arm, hazard ratio=0.71 (95% CI=0.56-0.88, p=0.002).
The PFS and OS results in patients with squamous cell histology suggested no advantage for pemetrexed over placebo.
There were no clinically relevant differences observed for the safety profile of pemetrexed within the histology subgroups.
JMEN: Kaplan Meier plots of progression-free survival (PFS) and overall survival pemetrexed versus placebo in patients with NSCLC other than predominantly squamous cell histology:
PARAMOUNT
A multicentre, randomised, double-blind, placebo-controlled Phase 3 study (PARAMOUNT), compared the efficacy and safety of continuation maintenance treatment with pemetrexed plus BSC (n=359) with that of placebo plus BSC (n=180) in patients with locally advanced (Stage IIIB) or metastatic (Stage IV) NSCLC other than predominantly squamous cell histology who did not progress after 4 cycles of first line doublet therapy of pemetrexed in combination with cisplatin. Of the 939 patients treated with pemetrexed plus cisplatin induction, 539 patients were randomised to maintenance treatment with pemetrexed or placebo. Of the randomised patients, 44.9% had a complete/partial response and 51.9% had a response of stable disease to pemetrexed plus cisplatin induction. Patients randomised to maintenance treatment were required to have an ECOG performance status 0 or 1. The median time from the start of pemetrexed plus cisplatin induction therapy to the start of maintenance treatment was 2.96 months on both the pemetrexed arm and the placebo arm. Randomised patients received maintenance treatment until disease progression. Efficacy and safety were measured from the time of randomisation after completion of first line (induction) therapy. Patients received a median of 4 cycles of maintenance treatment with pemetrexed and 4 cycles of placebo. A total of 169 patients (47.1%) completed ≥6 cycles maintenance treatment with pemetrexed, representing at least 10 total cycles of pemetrexed.
The study met its primary endpoint and showed a statistically significant improvement in PFS in the pemetrexed arm over the placebo arm (n=472, independently reviewed population; median of 3.9 months and 2.6 months, respectively) (hazard ratio=0.64, 95% CI=0.51-0.81, p=0.0002). The independent review of patient scans confirmed the findings of the investigator assessment of PFS. For randomised patients, as measured from the start of pemetrexed plus cisplatin first line induction treatment, the median investigator-assessed PFS was 6.9 months for the pemetrexed arm and 5.6 months for the placebo arm (hazard ratio=0.59 95% CI=0.47-0.74).
Following pemetrexed plus cisplatin induction (4 cycles), treatment with pemetrexed was statistically superior to placebo for OS (median 13.9 months versus 11.0 months, hazard ratio=0.78, 95%CI=0.64-0.96, p=0.0195). At the time of this final survival analysis, 28.7% of patients were alive or lost to follow up on the pemetrexed arm versus 21.7% on the placebo arm. The relative treatment effect of pemetrexed was internally consistent across subgroups (including disease stage, induction response, ECOG PS, smoking status, gender, histology and age) and similar to that observed in the unadjusted OS and PFS analyses. The 1 year and 2 year survival rates for patients on pemetrexed were 58% and 32% respectively, compared to 45% and 21% for patients on placebo. From the start of pemetrexed plus cisplatin first line induction treatment, the median OS of patients was 16.9 months for the pemetrexed arm and 14.0 months for the placebo arm (hazard ratio=0.78, 95% CI=0.64-0.96). The percentage of patients that received post study treatment was 64.3% for pemetrexed and 71.7% for placebo.
PARAMOUNT:Kaplan Meier plot of progression-free survival (PFS) and Overall Survival (OS) for continuation pemetrexed maintenance versus placebo in patients with NSCLC other than predominantly squamous cell histology (measured from randomisation):
The pemetrexed maintenance safety profiles from the two studies JMEN and PARAMOUNT were similar.
Pharmacokinetic properties
The pharmacokinetic properties of pemetrexed following single-agent administration have been evaluated in 426 cancer patients with a variety of solid tumours at doses ranging from 0.2 to 838 mg/m² infused over a 10 minute period. Pemetrexed has a steady-state volume of distribution of 9 l/m². In vitro studies indicate that pemetrexed is approximately 81% bound to plasma proteins. Binding was not notably affected by varying degrees of renal impairment. Pemetrexed undergoes limited hepatic metabolism. Pemetrexed is primarily eliminated in the urine, with 70% to 90% of the administered dose being recovered unchanged in urine within the first 24 hours following administration. In Vitro studies indicate that pemetrexed is actively secreted by OAT3 (organic anion transporter. Pemetrexed total systemic clearance is 91.8 ml/min and the elimination half-life from plasma is 3.5 hours in patients with normal renal function (creatinine clearance of 90 ml/min). Between patient variability in clearance is moderate at 19.3%. Pemetrexed total systemic exposure (AUC) and maximum plasma concentration increase proportionally with dose. The pharmacokinetics of pemetrexed are consistent over multiple treatment cycles.
The pharmacokinetic properties of pemetrexed are not influenced by concurrently administered cisplatin. Oral folic acid and intramuscular vitamin B12 supplementation do not affect the pharmacokinetics of pemetrexed.
Preclinical safety data
Administration of pemetrexed to pregnant mice resulted in decreased foetal viability, decreased foetal weight, incomplete ossification of some skeletal structures and cleft palate.
Administration of pemetrexed to male mice resulted in reproductive toxicity characterised by reduced fertility rates and testicular atrophy. In a study conducted in beagle dog by intravenous bolus injection for 9 months, testicular findings (degeneration/necrosis of the seminiferous epithelium) have been observed. This suggests that pemetrexed may impair male fertility. Female fertility was not investigated.
Pemetrexed was not mutagenic in either the in vitro chromosome aberration test in Chinese hamster ovary cells, or the Ames test. Pemetrexed has been shown to be clastogenic in the in vivo micronucleus test in the mouse.
Studies to assess the carcinogenic potential of pemetrexed have not been conducted.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.