ARNUITY ELLIPTA Inhalation powder Ref.[10751] Active ingredients: Fluticasone furoate
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
Fluticasone furoate is a synthetic trifluorinated corticosteroid with anti‑inflammatory activity. Fluticasone furoate has been shown in vitro to exhibit a binding affinity for the human glucocorticoid receptor that is approximately 29.9 times that of dexamethasone and 1.7 times that of fluticasone propionate. The clinical relevance of these findings is unknown.
The precise mechanism through which fluticasone furoate affects asthma symptoms is not known. Inflammation is an important component in the pathogenesis of asthma. Corticosteroids have been shown to have a wide range of actions on multiple cell types (e.g., mast cells, eosinophils, neutrophils, macrophages, lymphocytes) and mediators (e.g., histamine, eicosanoids, leukotrienes, cytokines) involved in inflammation. Specific effects of fluticasone furoate demonstrated in in vitro and in vivo models included activation of the glucocorticoid response element, inhibition of pro-inflammatory transcription factors such as NFkB, and inhibition of antigen-induced lung eosinophilia in sensitized rats. These anti-inflammatory actions of corticosteroids may contribute to their efficacy.
Though effective for the treatment of asthma, corticosteroids may not affect symptoms immediately. Individual patients will experience a variable time to onset and degree of symptom relief. Maximum benefit may not be achieved for 1 to 2 weeks or longer after starting treatment. When corticosteroids are discontinued, asthma stability may persist for several days or longer.
Trials in subjects with asthma have shown a favorable ratio between topical anti-inflammatory activity and systemic corticosteroid effects with recommended doses of orally inhaled fluticasone furoate. This is explained by a combination of a relatively high local anti-inflammatory effect, negligible oral systemic bioavailability (approximately 1.3%), and the minimal pharmacological activity of the metabolites detected in man.
12.2. Pharmacodynamics
The pharmacodynamics of fluticasone furoate was characterized in trials of fluticasone furoate given as a single component and also in trials of fluticasone furoate given in combination with vilanterol.
Hypothalamic-Pituitary-Adrenal Axis Effects
Healthy Subjects
Inhaled fluticasone furoate at repeat doses up to 400 mcg was not associated with statistically significant decreases in serum or urinary cortisol in healthy subjects. Decreases in serum and urine cortisol levels were observed at fluticasone furoate exposures several-fold higher than exposures observed at the therapeutic dose.
Subjects with Asthma
A randomized, double-blind, parallel-group trial in 104 pediatric subjects with asthma (aged 5 to 11 years) showed no difference between once-daily treatment with ARNUITY ELLIPTA 50 mcg compared with placebo on serum cortisol weighted mean (0 to 24 hours) and serum cortisol AUC(0-24) following 6 weeks of treatment.
A randomized, double-blind, parallel-group trial in 185 subjects with asthma aged 12 to 65 years showed no difference between once-daily treatment with fluticasone furoate/vilanterol 100 mcg/25 mcg or fluticasone furoate/vilanterol 200 mcg/25 mcg compared with placebo on serum cortisol weighted mean (0 to 24 hours), serum cortisol AUC(0-24), and 24-hour urinary cortisol after 6 weeks of treatment, whereas prednisolone 10 mg given once daily for 7 days resulted in significant cortisol suppression.
Cardiac Electrophysiology
A QT/QTc trial did not demonstrate an effect of fluticasone furoate administration on the QTc interval. The effect of a single dose of 4,000 mcg of orally inhaled fluticasone furoate on the QTc interval was evaluated over 24 hours in 40 healthy male and female subjects in a placebo- and positive-controlled (a single dose of 400 mg oral moxifloxacin) cross-over trial. The QTcF maximal mean change from baseline following fluticasone furoate was similar to that observed with placebo with a treatment difference of 0.788 msec (90% CI: -1.802, 3.378). In contrast, moxifloxacin given as a 400-mg tablet resulted in prolongation of the QTcF maximal mean change from baseline compared with placebo with a treatment difference of 9.929 msec (90% CI: 7.339, 12.520).
12.3. Pharmacokinetics
The pharmacokinetics of fluticasone furoate was characterized in trials of fluticasone furoate given as a single component and in trials of fluticasone furoate given in combination with vilanterol. Linear pharmacokinetics was observed for fluticasone furoate (200 to 800 mcg). On repeated once-daily inhalation administration, steady state of fluticasone furoate plasma concentration was achieved after 6 days, and the accumulation was up to 2.6-fold as compared with single dose.
Absorption
Fluticasone furoate plasma levels may not predict therapeutic effect. Peak plasma concentrations are reached within 0.5 to 1 hour. Absolute bioavailability of fluticasone furoate when administrated by inhalation was 13.9%, primarily due to absorption of the inhaled portion of the dose delivered to the lung. Oral bioavailability from the swallowed portion of the dose is low (approximately 1.3%) due to extensive first-pass metabolism. Systemic exposure (AUC) in subjects with asthma was 26% lower than observed in healthy subjects.
Distribution
Following intravenous administration to healthy subjects, the mean volume of distribution at steady state was 661 L. Binding of fluticasone furoate to human plasma proteins was high (99.6%).
Metabolism
Fluticasone furoate is cleared from systemic circulation principally by hepatic metabolism via CYP3A4 to metabolites with significantly reduced corticosteroid activity. There was no in vivo evidence for cleavage of the furoate moiety resulting in the formation of fluticasone.
Elimination
Fluticasone furoate and its metabolites are eliminated primarily in the feces, accounting for approximately 101% and 90% of the orally and intravenously administered doses, respectively. Urinary excretion accounted for approximately 1% and 2% of the orally and intravenously administered doses, respectively. Following repeat-dose inhaled administration, the plasma elimination phase half-life averaged 24 hours.
Specific Populations
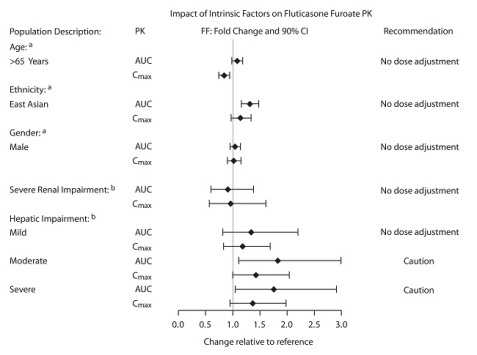
The effect of renal and hepatic impairment and other intrinsic factors on the pharmacokinetics of fluticasone furoate is shown in Figure 1.
Figure 1. Impact of Intrinsic Factors on the Pharmacokinetics (PK) of Fluticasone Furoate (FF):
a Age, gender, and ethnicity comparison for ARNUITY ELLIPTA in adult and adolescent subjects with asthma.
b Renal groups (fluticasone furoate/vilanterol 200 mcg/25 mcg) and hepatic groups (fluticasone furoate/vilanterol 200 mcg/25 mcg or fluticasone furoate/vilanterol 100 mcg/12.5 mcg) compared with healthy control group.
Pediatric Patients
A population pharmacokinetics analysis to assess impact of age on fluticasone furoate systemic exposure was conducted using combined data from clinical trials in pediatric subjects aged 5 to 11 years (n=306). There was no relevant effect of age on the apparent clearance of fluticasone furoate. The rate and extent of fluticasone furoate systemic exposure at steady state in children aged 5 to 11 years were comparable to that observed in adult and adolescent subjects following dosing with fluticasone furoate 100 mcg monotherapy.
Racial or Ethnic Groups
Systemic exposure (AUC(0-24)) to inhaled fluticasone furoate 200 mcg was 27% to 49% higher in healthy subjects of Japanese, Korean, and Chinese heritage compared with Caucasian subjects. Similar differences were observed for subjects with asthma (Figure 1). However, there is no evidence that this higher exposure to fluticasone furoate results in clinically relevant effects on urinary cortisol excretion or on efficacy in these racial groups.
Patients with Hepatic Impairment
Following repeat dosing of fluticasone furoate/vilanterol 200 mcg/25 mcg (100 mcg/12.5 mcg in the severe impairment group) for 7 days, there was an increase of 34%, 83%, and 75% in fluticasone furoate systemic exposure (AUC) in subjects with mild, moderate, and severe hepatic impairment, respectively, compared with healthy subjects (Figure 1).
In subjects with moderate hepatic impairment receiving fluticasone furoate/vilanterol 200 mcg/25 mcg, mean serum cortisol (0 to 24 hours) was reduced by 34% (90% CI: 11%, 51%) compared with healthy subjects. In subjects with severe hepatic impairment receiving fluticasone furoate/vilanterol 100 mcg/12.5 mcg, mean serum cortisol (0 to 24 hours) was increased by 14% (90% CI: -16%, 55%) compared with healthy subjects. Patients with moderate to severe hepatic disease should be closely monitored.
Patients with Renal Impairment
Fluticasone furoate systemic exposure was not increased in subjects with severe renal impairment compared with healthy subjects (Figure 1). There was no evidence of greater corticosteroid class-related systemic effects (assessed by serum cortisol) in subjects with severe renal impairment compared with healthy subjects.
Drug Interaction Studies
The potential for fluticasone furoate to inhibit or induce metabolic enzymes and transporter systems is negligible at low inhalation doses.
Inhibitors of Cytochrome P450 3A4
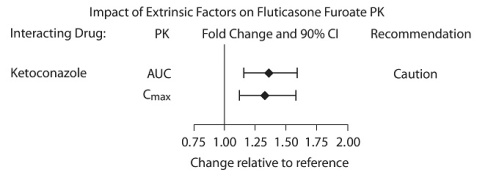
The exposure (AUC) of fluticasone furoate was 36% higher after single and repeated doses when coadministered with ketoconazole 400 mg compared with placebo (Figure 2). The increase in fluticasone furoate exposure was associated with a 27% reduction in weighted mean serum cortisol (0 to 24 hours).
Figure 2. Impact of Coadministered Ketoconazolea on the Pharmacokinetics (PK) of Fluticasone Furoate
a Compared with placebo group.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Fluticasone furoate produced no treatment-related increases in the incidence of tumors in 2-year inhalation studies in rats and mice at inhaled doses up to 9 and 19 mcg/kg/day, respectively (less than the MRHDID on a mcg/m² basis).
Fluticasone furoate did not induce gene mutation in bacteria or chromosomal damage in a mammalian cell mutation test in mouse lymphoma L5178Y cells in vitro. There was also no evidence of genotoxicity in the in vivo micronucleus test in rats.
No evidence of impairment of fertility was observed in male and female rats at inhaled fluticasone furoate doses up to 29 and 91 mcg/kg/day, respectively (approximately 1 and 4 times, respectively, the MRHDID in adults on a mcg/m² basis).
14. Clinical Studies
The safety and efficacy of ARNUITY ELLIPTA were evaluated in 3,611 adult and adolescent subjects with asthma. The development program included 4 confirmatory trials of 3 and 6 months' duration and 3 dose-ranging trials of 8 weeks' duration. The efficacy of ARNUITY ELLIPTA is based primarily on the dose-ranging trials and the confirmatory trials described below. One additional trial evaluated the safety and efficacy of ARNUITY ELLIPTA in 593 subjects aged 5 to 11 years.
14.1 Dose-Ranging Trials
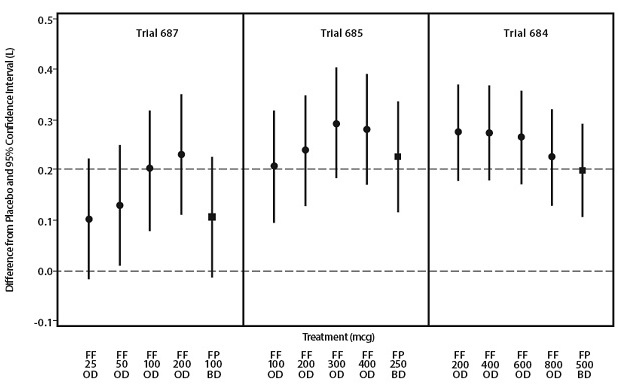
Eight doses of fluticasone furoate ranging from 25 to 800 mcg once daily were evaluated in 3 randomized, double-blind, placebo-controlled, 8-week trials in adult and adolescent subjects with asthma. Across the 3 trials, subjects were uncontrolled at baseline on treatments of short-acting beta2-agonist and/or non-corticosteroid controller medications (Trial 687), low-dose ICS (Trial 685), or medium doses of ICS (Trial 684). The trials in Figure 3 were dose-ranging trials of ARNUITY ELLIPTA not designed to provide comparative effectiveness data and should not be interpreted as evidence of superiority/inferiority to fluticasone propionate. A dose-related increase in trough FEV1 at Week 8 was seen for doses from 25 to 200 mcg with no consistent additional benefit for doses above 200 mcg as seen in Figure 3. To evaluate dosing frequency, a separate trial compared fluticasone furoate 200 mcg once daily, fluticasone furoate 100 mcg twice daily, fluticasone propionate 100 mcg twice daily, and fluticasone propionate 200 mcg once daily. The results supported the selection of the once-daily dosing frequency.
Figure 3. Dose-Ranging Trials:
FF = Fluticasone furoate, FP = Fluticasone propionate, OD = Once daily, BD = Twice daily.
14.2 Confirmatory Trials
Adult and Adolescent Subjects Aged 12 Years and Older
The clinical development program for ARNUITY ELLIPTA included 4 confirmatory trials in adult and adolescent subjects with asthma aged 12 years and older. The trials were designed to evaluate the safety and efficacy of ARNUITY ELLIPTA given once daily in the evening on lung function in subjects who were not controlled on their current treatments of ICS, or combination therapy consisting of an ICS plus a LABA. Study treatments were delivered as inhalation powders. The primary endpoint in all trials was change from baseline in evening trough FEV1 measured approximately 24 hours after the final dose of study medication. Trough FEV1 (assessed at approximately 24 hours after the previous dose) was also assessed at clinic visits throughout the trials. Trials 2 and 4 had a co-primary endpoint of change from baseline in weighted mean serial FEV1 measured after the final dose of study medication at 5, 15, and 30 minutes and 1, 2, 3, 4, 5, 12, 16, 20, 23, and 24 hours post-dose.
Clinical Trials with ARNUITY ELLIPTA 100 mcg
Trial 1 was a 24-week trial that evaluated the efficacy of ARNUITY ELLIPTA 100 mcg compared with placebo on lung function in subjects with asthma. Inhaled fluticasone propionate 250 mcg twice daily was included as an active control. Of the 343 subjects, 59% were female and 79% were Caucasian. The mean age was 41 years. The trial included a 4-week run-in period during which the subjects were symptomatic while taking their usual low- to mid-dose ICS therapy (i.e., fluticasone propionate 100 to 500 mcg daily or equivalent). Mean baseline percent predicted FEV1 was approximately 73% overall and was similar across the 3 treatment groups. Thirty-five percent of subjects on placebo and 19% of subjects on ARNUITY ELLIPTA 100 mcg failed to complete the 24-week trial.
The change in trough FEV1 from baseline to Week 24, or the last available on-treatment visit prior to Week 24, was assessed to evaluate the efficacy of ARNUITY ELLIPTA 100 mcg. The mean change from baseline in trough FEV1 was greater among subjects receiving ARNUITY ELLIPTA 100 mcg than among those receiving placebo (mean treatment difference from placebo 146 mL; 95% CI: 36, 257) as shown in Table 3.
Table 3. Change from Baseline in Trough FEV1 (mL) at Week 24 – Trial 1:
| Trough FEV1 (Week 24) | Placebo (n=113) | ARNUITY ELLIPTA 100 mcg (n=111) | Fluticasone Propionate 250 mcg Twice Daily (n=107) |
|---|---|---|---|
| Least squares mean | 2,372 | 2,519 | 2,517 |
| Least squares mean change (SE) | 15 (39.4) | 161 (39.8) | 159 (40.6) |
| Column vs. placebo | |||
| Difference | — | 146 | 145 |
| 95% CI | — | 36, 257 | 33, 257 |
| em>P value | — | 0.009 | 0.011 |
FEV1 = forced expiratory volume in 1 second, SE = standard error, CI = confidence interval.
Trial 2 was a 12-week trial that evaluated the efficacy of ARNUITY ELLIPTA 100 mcg on lung function in subjects with asthma compared with placebo. The combination of fluticasone furoate 100 mcg and vilanterol 25 mcg was also included as a treatment arm. Of the 609 subjects, 58% were female and 84% were Caucasian. The mean age was 40 years. The trial included a 4-week run-in period during which the subjects were symptomatic while taking their usual low- to mid-dose ICS (fluticasone propionate 200 to 500 mcg/day or equivalent). If LABA were used prior to screening, their use was discontinued during the run-in. Mean baseline percent predicted FEV1 was approximately 70% in both treatment groups. Twenty-six percent of subjects on placebo and 10% of subjects on ARNUITY ELLIPTA 100 mcg failed to complete the 12-week trial.
The co-primary efficacy endpoints in Trial 2 were change from baseline in trough FEV1 at Week 12 and weighted mean FEV1 (0-24 hours) at the end of the 12-week treatment period. Trough FEV1 was assessed at clinic visits throughout the trial. Weighted mean FEV1 (0-24 hours) was recorded at baseline and after the final study dose with serial measurements taken at frequent intervals (at 5, 15, and 30 minutes and 1, 2, 3, 4, 5, 12, 16, 20, 23, and 24 hours post-dose) in a subset of subjects (n=201).
ARNUITY ELLIPTA 100 mcg once daily had greater mean changes from baseline in trough FEV1 than placebo throughout the trial. At Week 12 or the last available on-treatment visit prior to Week 12, the mean change from baseline in trough FEV1 was greater among subjects receiving ARNUITY ELLIPTA 100 mcg once daily than among those receiving placebo (mean treatment difference 136 mL; 95% CI: 51, 222).
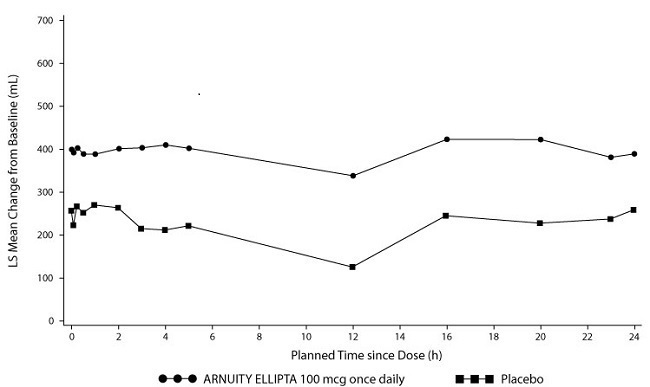
Lung function improvements were sustained over the 24-hour period following the final dose of ARNUITY ELLIPTA 100 mcg (Figure 4). Compared with placebo, at Week 12 the change from baseline in weighted mean FEV1 was significantly greater for ARNUITY ELLIPTA 100 mcg (mean treatment difference 186 mL; 95% CI: 62, 310).
Figure 4. Mean Change from Baseline in Individual Serial FEV1 (mL) Assessments after 12 Weeks of Treatment – Trial 2:
Subjects in both Trials 1 and 2 receiving ARNUITY ELLIPTA 100 mcg once daily had a greater improvement from baseline in percentage of 24-hour periods without need of beta2-agonist rescue medication use than subjects receiving placebo.
Clinical Trial with ARNUITY ELLIPTA 200 mcg
Trial 3 was a 24-week trial that evaluated the relative efficacy of ARNUITY ELLIPTA 100 mcg and ARNUITY ELLIPTA 200 mcg on lung function in subjects with asthma. Of the 219 subjects, 68% were female and 87% were Caucasian. The mean age was 46 years. The trial included a 4-week run-in period during which the subjects were symptomatic while taking their usual mid- to high-dose ICS therapy (i.e., fluticasone propionate greater than 250 to 1,000 mcg/day or equivalent). If LABA were used prior to screening, their use was discontinued during the run-in. Mean baseline percent predicted FEV1 was approximately 68% overall and similar in the 2 treatment groups. Sixteen percent of subjects on ARNUITY ELLIPTA 100 mcg and 13% of subjects on ARNUITY ELLIPTA 200 mcg failed to complete the 24-week trial.
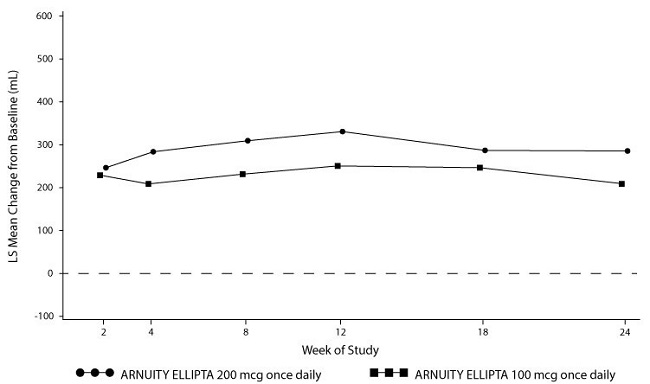
The primary efficacy endpoint was mean change from baseline in trough FEV1 at Week 24. There were trends toward greater mean changes from baseline in the group receiving ARNUITY ELLIPTA 200 mcg than the group receiving ARNUITY ELLIPTA 100 mcg throughout the trial (Figure 5). At Week 24 or the last available on-treatment visit prior to Week 24, the mean change from baseline in trough FEV1 was 208 mL for ARNUITY ELLIPTA 100 mcg, as compared with 284 mL for ARNUITY ELLIPTA 200 mcg (difference of 77 mL; 95% CI: -39, 192) as seen in Figure 5.
Figure 5. Mean Change from Baseline in Trough FEV1 (mL) over Time – Trial 3:
Trial 4 was a 24-week trial that evaluated the efficacy of ARNUITY ELLIPTA 200 mcg once daily and fluticasone propionate 500 mcg twice daily on lung function in subjects with asthma. The combination of fluticasone furoate 200 mcg and vilanterol 25 mcg was also included as a treatment arm (data not shown). Of the 586 subjects, 59% were female and 84% were Caucasian. The mean age was 46 years. The trial included a 4-week run-in period during which the subjects were symptomatic while taking their usual mid- to high-dose ICS (fluticasone propionate 500 to 1,000 mcg/day or equivalent). If LABA were used prior to screening, their use was discontinued during the run-in. Mean baseline percent predicted FEV1 was approximately 67% in both treatment groups.
Both ARNUITY ELLIPTA 200 mcg once daily and fluticasone propionate 500 mcg twice daily produced improvement from baseline in lung function. At Week 24 the mean change from baseline in trough FEV1 was 201 mL for ARNUITY ELLIPTA 200 mcg once daily and 183 mL for fluticasone propionate 500 mcg twice daily (treatment difference of 18 mL, 95% CI: -66, 102).
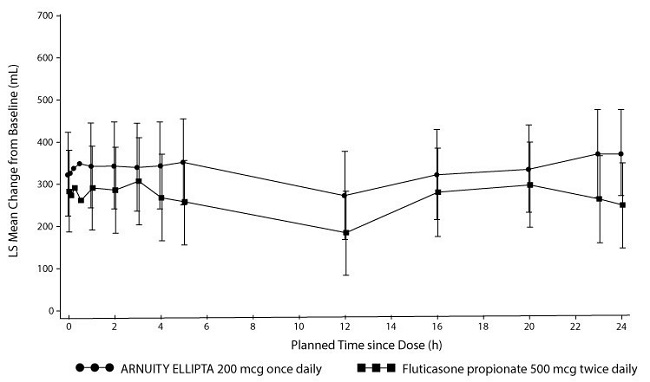
Lung function improvements were sustained over the 24-hour period following the final dose of ARNUITY ELLIPTA 200 mcg (Figure 6). At Week 24, the change from baseline in weighted mean FEV1 was 328 mL for ARNUITY ELLIPTA 200 mcg once daily and 258 mL for fluticasone propionate 500 twice daily (difference of 70 mL; 95% CI: -67, 208).
Figure 6. Mean Change from Baseline in Individual Serial FEV1 (mL) Assessments after 24 Weeks of Treatment – Trial 4:
Pediatric Subjects Aged 5 to 11 Years
A 12-week trial evaluated the efficacy of fluticasone furoate (25, 50, or 100 mcg) administered once daily in the evening compared with placebo in 593 pediatric subjects with asthma aged 5 to 11 years. Inhaled fluticasone propionate 100 mcg twice daily was included as an active control. At trial entry subjects were symptomatic, had at least a 6-month history of asthma, and had been receiving stable asthma therapy for at least 4 weeks prior to screening. Subjects had to have a pre-bronchodilator PEF of ≥60% to ≤90% of their best post-bronchodilator value and, in subjects able to perform the maneuver, demonstrate a ≥12% reversibility of FEV1 within approximately 10 to 40 minutes following 2 to 4 inhalations of albuterol inhalation aerosol. The primary endpoint of this trial was the mean change from baseline in daily pre-dose AM PEF from the patient electronic daily diary averaged over the 12-week treatment period. A secondary endpoint was the change from baseline in the percentage of rescue-free 24-hour periods during the 12-week treatment period. Of the 593 subjects, the mean age was 8 years, 62% were male, and 42% were Caucasian. Lung function improvements based on the primary endpoint of mean change from baseline in AM PEF are presented in Table 4.
Table 4. Least Squares Mean Change from Baseline in Pre-Dose AM PEF over the 12-Week Treatment Period (Intent-to-Treat Population):
| Primary Endpoint | Placebo (n=119) | Fluticasone Furoate 25 mcg (n=118) | Fluticasone Furoate 50 mcg (n=120) | Fluticasone Furoate 100 mcg (n=118) | Fluticasone Propionate 100 mcg (n=118) |
|---|---|---|---|---|---|
| AM PEF (L/min)a | n=119 | n=117 | n=118 | n=118 | n=117 |
| LS mean change (SE) | 3.3 (2.63) | 21.9 (2.66) | 22.8 (2.65) | 15.8 (2.64) | 17.3 (2.64) |
| Difference vs placebo | 18.6 | 19.5 | 12.5 | 14.0 | |
| (95% CI) | (11.3, 26.0) | (12.1, 26.9) | (5.1, 19.8) | (6.7, 21.4) |
AM PEF = Morning Peak Expiratory Flow, LS = Least squares, SE = standard error, CI = confidence interval.
a Average over Weeks 1 to 12.
Pediatric subjects receiving ARNUITY ELLIPTA 50 mcg had a greater improvement from baseline in percentage of 24-hour periods without need of beta2-agonist rescue medication use than subjects receiving placebo.
Given the demonstration of efficacy of ARNUITY ELLIPTA 100 mcg and ARNUITY ELLIPTA 200 mcg in the adult and adolescent population, the results support the efficacy of ARNUITY ELLIPTA 50 mcg once daily in pediatric subjects with asthma aged 5 to 11 years.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.