ARZERRA Concentrate for solution for infusion Ref.[8957] Active ingredients: Ofatumumab
Source: European Medicines Agency (EU) Revision Year: 2017 Publisher: Novartis Europharm Limited, Frimley Business Park, Camberley GU16 7SR, United Kingdom
Pharmacodynamic properties
Pharmacotherapeutic group: antineoplasic agents, monoclonal antibodies
ATC code: L01XC10
Mechanism of action
Ofatumumab is a human monoclonal antibody (IgG1) that binds specifically to a distinct epitope encompassing both the small and large extracellular loops of the CD20 molecule. The CD20 molecule is a transmembrane phosphoprotein expressed on B lymphocytes from the pre-B to mature B lymphocyte stage and on B-cell tumours. The B-cell tumours include CLL (generally associated with lower levels of CD20 expression) and non-Hodgkin’s lymphomas (where >90% of tumours have high levels of CD20 expression). The CD20 molecule is not shed from the cell surface and is not internalised following antibody binding.
The binding of ofatumumab to the membrane-proximal epitope of the CD20 molecule induces recruitment and activation of the complement pathway at the cell surface, leading to complementdependent cytotoxicity and resultant lysis of tumour cells. Ofatumumab has been shown to induce appreciable lysis of cells with high expression levels of complement defence molecules. Ofatumumab has also been shown to induce cell lysis in both high and low CD20 expressing cells and in rituximabresistant cells. In addition, the binding of ofatumumab allows the recruitment of natural killer cells allowing the induction of cell death through antibody-dependent cell-mediated cytotoxicity.
Pharmacodynamic effects
Peripheral B-cell counts decreased after the first ofatumumab infusion in patients with haematological malignancies. In all patients with CLL, ofatumumab induces rapid and profound B-cell depletion, whether given as a single agent or in combination.
When ofatumumab was administered as single agent in patients with refractory CLL, the median decrease in B-cell counts was 22% after the first infusion and 92% at the eighth weekly infusion. Peripheral B-cell counts remained low throughout the remainder of therapy in most patients and remained below baseline up to 15 months after the last dose in patients who responded.
When ofatumumab was administered in combination with chlorambucil in patients with previously untreated CLL, the median decreases in B-cell counts after the first cycle and prior to the sixth monthly cycle were 94% and >99%. At 6 months after the last dose, the median reductions in B-cell counts were >99%.
When ofatumumab was administered in combination with fludarabine and cyclophosphamide in patients with relapsed CLL, the median decrease from baseline was 60% after the first infusion and complete depletion (100%) was reached after 4 cycles.
Immunogenicity
There is a potential for immunogenicity with therapeutic proteins such as ofatumumab. Serum samples from more than 1,000 patients across the CLL clinical programme were tested for anti-ofatumumab antibodies during and after treatment periods ranging from 8 weeks to 2 years. Formation of anti-ofatumumab antibodies was observed for less than 0.5% of patients with CLL after treatment with ofatumumab.
Clinical efficacy and safety
Previously untreated CLL
Study OMB110911 (randomised, open-label, parallel-arm, multicentre) evaluated the efficacy of Arzerra in combination with chlorambucil compared with chlorambucil alone in 447 patients with previously untreated CLL considered inappropriate for fludarabine-based treatment (e.g. due to advanced age or presence of co-morbidities), with active disease and indicated for treatment. Patients received either Arzerra as monthly intravenous infusions (cycle 1: 300 mg on day 1 and 1000 mg on day 8; subsequent cycles: 1000 mg on day 1 every 28 days) in combination with chlorambucil (10 mg/m² orally on days 1-7 every 28 days) or chlorambucil alone (10 mg/m² orally on days 1-7 every 28 days). Patients received treatment for a minimum of 3 months until best response or up to a maximum of 12 cycles. The median age was 69 years (range: 35 to 92 years), 27% patients were ≥75 years of age, 63% were male and 89% were white. Median Cumulative Illness Rating Score for Geriatrics (CIRS-G) was 9 and 31% of patients had a CIRS-G >10. Median creatinine clearance (CrCl), assessed with the use of the Cockroft-Gault formula, was 70 ml/min and 48% of patients had a CrCl of <70 ml/min. Patients with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2 were enrolled into the study and 91% had an ECOG performance status of 0 or 1. Approximately 60% of patients received 3-6 cycles of Arzerra and 32% received 7-12 cycles. The median number of cycles completed in patients was 6 (total Arzerra dose of 6300 mg).
The primary endpoint was median progression-free survival (PFS) as assessed by a blinded Independent Review Committee (IRC) using the International Workshop for Chronic Lymphocytic Leukaemia (IWCLL) updated National Cancer Institute-sponsored Working Group (NCI-WG) guidelines (2008). The overall response rate (ORR) including complete response (CR) was also assessed by an IRC using the 2008 IWCLL guidelines.
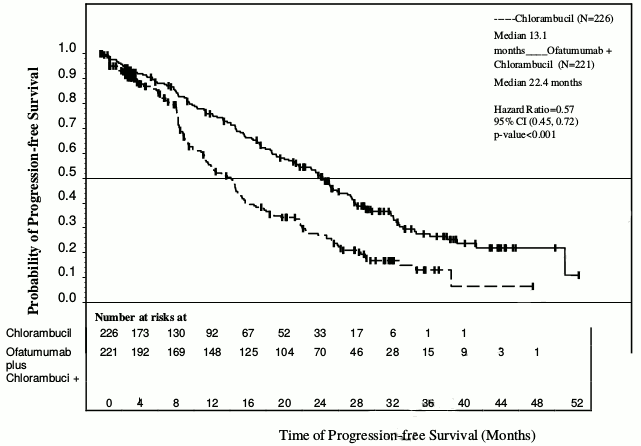
Arzerra in combination with chlorambucil showed a statistically significant (71%) improvement in median PFS compared with chlorambucil alone (HR: 0.57; 95% CI: 0.45, 0.72) (see Table 1 and Figure 1). PFS benefit with the addition of Arzerra was observed in all patients, including those with poor-risk biological features (such as 17p or 11q deletion, unmutated IGHV, β2M >3500 μg/l, and ZAP-70 expression).
Table 1. Summary of PFS with Arzerra in combination with chlorambucil compared with chlorambucil in previously untreated CLL:
| IRC-assessed primary and subgroup analyses of PFS, months | Chlorambucil (N=226) | Arzerra and chlorambucil (N=221) |
|---|---|---|
| Median, all patients | 13.1 | 22.4 |
| 95% CI | (10.6, 13.8) | (19.0, 25.2) |
| Hazard ratio | 0.57 (0.45, 0.72) | |
| P value | p<0.001 | |
| Age ≥75 years (n=119) | 12.2 | 23.8 |
| Co-morbidity 0 or 1 (n=126) | 10.9 | 23.0 |
| Co-morbidity 2 or more (n=321) | 13.3 | 21.9 |
| ECOG 0, 1 (n=411) | 13.3 | 23.0 |
| ECOG 2 (n=35) | 7.9 | 20.9 |
| CIRS-G ≤10 (n=310) | 13.1 | 21.7 |
| CIRS-G >10 (n=137) | 12.2 | 23.2 |
| CrCl <70 ml/min (n=214) | 10.9 | 23.1 |
| CrCl ≥70 ml/min (n=227) | 14.5 | 22.1 |
| 17p or 11q deletion (n=90) | 7.9 | 13.6 |
| IGHV mutated (≤98%) (n=177) | 12.2 | 30.5 |
| IGHV unmutated (>98%) (n=227) | 11.7 | 17.3 |
| β2M ≤3500 μg/l (n=109) | 13.8 | 25.5 |
| β2M >3500 μg/l (n=322) | 11.6 | 19.6 |
| ZAP-70 positive (n=161) | 9.7 | 17.7 |
| ZAP-70 intermediate (n=160) | 13.6 | 25.3 |
| ZAP-70 negative (n=100) | 13.8 | 25.6 |
| IGHV mutated & ZAP-70 negative (n=60) | 10.5 | NR |
| IGHV mutated & ZAP-70 positive (n=35) | 7.9 | 27.2 |
| IGHV unmutated & ZAP-70 negative (n=27) | 16.7 | 16.2 |
| IGHV unmutated & ZAP-70 positive (n=122) | 11.2 | 16.2 |
Abbreviations: β2M = beta-2-microglobulin, CI = confidence interval; CIRS-G = Cumulative Illness Rating Scale for Geriatrics, CLL = chronic lymphocytic leukaemia, CrCl = creatinine clearance, ECOG = Eastern Cooperative Oncology Group, IGHV = Immunoglobulin Heavy Chain Variable Region, IRC = Independent Review Committee, N = number, NR = not reached, PFS = progression-free survival, ZAP-70 = zeta-chain-associated protein kinase 70.
Limited data are available in the heterogeneous non-white population and in patients with an ECOG performance status of PS = 2.
Figure 1. Kaplan-Meier estimates of IRC-assessed PFS in previously untreated CLL:
Table 2. Summary of secondary outcomes of Arzerra in combination with chlorambucil compared with chlorambucil in previously untreated CLL:
| IRC-assessed secondary outcome | Chlorambucil (N=226) | Arzerra and chlorambucil (N=221) |
|---|---|---|
| ORR (%) | 69 | 82 |
| 95% CI | (62.1, 74.6) | (76.7, 87.1) |
| P value | p<0.001 | |
| CR (%) | 1 | 12 |
| CR with MRD negativity (% of CR) | 0 | 37 |
| Median duration of response, all patients, months | 13.2 | 22.1 |
| 95% CI | (10.8, 16.4) | (19.1, 24.6) |
| P value | p<0.001 | |
Abbreviations: CI = confidence interval, CLL = chronic lymphocytic leukaemia, CR = complete response, IRC = Independent Review Committee, MRD = minimal residue disease, N = number, ORR = overall response rate
Study OMB115991 evaluated the efficacy of Arzerra in combination with bendamustine in 44 patients with previously untreated CLL considered inappropriate for fludarabine-based treatment. Patients received Arzerra as monthly intravenous infusions (cycle 1 300 mg on day 1 and 1000 mg on day 8; subsequent cycles: 1000 mg on day 1 every 28 days) in combination with intravenous bendamustine 90 mg/m² on days 1 and 2 every 28 days. Patients received treatment for a maximum of 6 cycles. The median number of cycles completed in patients was 6 (total Arzerra dose of 6300 mg).
The primary endpoint was ORR assessed by the investigator according to the 2008 IWCLL guidelines.
The results of this study demonstrated that Arzerra in combination with bendamustine is an effective therapy providing an ORR of 95% (95% CI: 85, 99) and a CR of 43%. More than half of the patients (56%) with CR were MRD negative following the completion of study treatment.
No data comparing Arzerra in combination with bendamustine or with chlorambucil versus a rituximab based regimen such as rituximab with chlorambucil is available. Thus, the benefit of such a new combination over a rituximab based regimen is unknown.
Relapsed CLL
Study OMB110913 (randomised, open-label, parallel-arm, multicentre trial) evaluated the efficacy of ofatumumab in combination with fludarabine and cyclophosphamide compared with fludarabine and cyclophosphamide in 365 patients with relapsed CLL (defined as a patient who has received at least one prior CLL therapy and previously achieved a complete or partial remission/response, but after a period of six or more months demonstrated evidence of disease progression). Baseline disease characteristics and prognostic markers were balanced between treatment arms and representative of a relapsed CLL population. Patient median age was 61 years (range: 32 to 90 years, 7% were 75 years of age or older), 60% were male and 16%, 55% and 28% of patients were Binet stage A, B and C, respectively. The majority of patients (81%) received 1-2 prior lines of treatments (of whom approximately 50% received 1 prior treatment) and 21% of patients had received prior rituximab. The median CIRS score was 7 (range: 4 to 17), 36% of patients had CrCL <70 ml/min, 93% of patients had ECOG 0 or 1. Limited data are available in the heterogeneous non-white population and in patients with an ECOG performance status of 2.
Patients received ofatumumab as intravenous infusions (cycle 1: 300 mg on day 1 and 1000 mg on day 8; subsequent cycles: 1000 mg on day 1 every 28 days). Approximately 90% of patients received 3-6 cycles of ofatumumab and 66% completed 6 cycles.
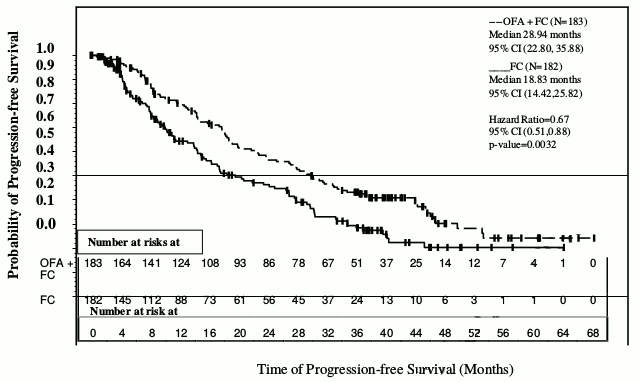
The primary endpoint of progression-free survival (PFS), as assessed by a blinded independent review committee (IRC) using the updated National Cancer Institute-sponsored Working Group (NCI-WG) guidelines (2008), was prolonged in the ofatumumab plus fludarabine-cyclophosphamide (OFA+FC) arm compared to the fludarabine-cyclophosphamide (FC) arm (28.9 months versus 18.8 months; HR: 0.67; 95% CI: 0.51-0.88, p=0.0032) resulting in a 10-month improvement in median PFS (see Figure 2). PFS based on local (investigator) assessment was consistent with the primary endpoint and resulted in a ~11-month improvement in median PFS (OFA+FC 27.2 months versus 16.8 months for FC; HR=0.66 (95% CI: 0.51, 0.85, p=0.0009).
Figure 2. Kaplan-Meier estimates of PFS in relapsed CLL:
The overall response rate (ORR) was also assessed by an IRC using the 2008 NCI-WG guidelines. The ORR was higher for OFA+FC versus FC (84% versus 68%, p=0.0003). Median time to next therapy was longer for the OFA+FC arm versus FC (48.1 months versus 40.1 months; HR: 0.73; 95% CI: 0.51-1.05). Median time to progression was longer for the OFA+FC arm versus FC (42.1 months versus 26.8 months; HR: 0.63; 95% CI: 0.45-0.87).
With a median follow-up of approximately 34 months, 67 deaths (37%) in the OFA+FC arm and 69 deaths (38%) in the FC arm were reported. The overall survival results showed a HR=0.78 (56.4 months versus 45.8 months for the OFA+FC arm versus FC arm; 95% CI: 0.56-1.09; p=0.1410).
Refractory CLL
Arzerra was administered as monotherapy to 223 patients with refractory CLL (study Hx-CD20-406). Patient median age was 64 years (range: 41 to 87 years), and the majority were male (73%) and white (96%). Patients received a median of 5 prior therapies, including rituximab (57%). Of these 223 patients, 95 patients were refractory to fludarabine and alemtuzumab therapy (defined as failure to achieve at least a partial response with fludarabine or alemtuzumab treatment or disease progression within 6 months of the last dose of fludarabine or alemtuzumab). Baseline cytogenetic (FISH) data were available for 209 patients. 36 patients had a normal karyotype and chromosomal aberrations were detected in 174 patients; there were 47 patients with 17p deletion, 73 patients with 11q deletion, 23 patients with trisomy 12q, and 31 patients with 13q deletion as the sole aberration.
The ORR was 49% in patients refractory to fludarabine and alemtuzumab (see Table 3 for a summary of the efficacy data from the study). Patients who had prior rituximab therapy, either as monotherapy or in combination with other medicinal products, responded to treatment with Arzerra at a similar rate to those who had not had prior rituximab therapy.
Table 3. Summary of response to Arzerra in patients with refractory CLL:
| (Primary) endpoint1 | Patients refractory to fludarabine and alemtuzumab n=95 |
|---|---|
| Overall response rate | |
| Responders, n (%) | 47 (49) |
| 95.3% CI (%) | 39, 60 |
| Response rate in patients with prior rituximab therapy | |
| Responders, n (%) | 25/56 (45) |
| 95% CI (%) | 31, 59 |
| Response rate in patients with chromosomal abnormality | |
| 17p deletion | |
| Responders, n (%) | 10/27 (37) |
| 95% CI (%) | 19, 58 |
| 11q deletion | |
| Responders, n (%) | 15/32 (47) |
| 95% CI (%) | 29, 65 |
| Median overall survival | |
| Months | 13.9 |
| 95% CI | 9.9, 18.6 |
| Progression-free survival | |
| Months | 4.6 |
| 95% CI | 3.9, 6.3 |
| Median duration of response | |
| Months | 5.5 |
| 95% CI | 3.7, 7.2 |
| Median time to next CLL therapy | |
| Months | 8.5 |
| 95% CI | 7.2, 9.9 |
1 The overall response was assessed by an Independent Response Committee using the 1996 NCI-WG guidelines for CLL.
Improvements also were demonstrated in components of the NCI-WG response criteria. These included improvements associated with constitutional symptoms, lymphadenopathy, organomegaly, or cytopenias (see Table 4).
Table 4. Summary of clinical improvement with a minimum duration of 2 months in refractory patients with abnormalities at baseline:
| Patients with benefit/patients with abnormality at baseline (%) | |
|---|---|
| Efficacy endpoint or haematological parametera | Patients refractory to fludarabine and alemtuzumab |
| Lymphocyte count | |
| ≥50% decrease | 49/71 (69) |
| Normalisation (≤4x109/l) | 36/71 (51) |
| Complete resolution of constitutional symptomsb | 21/47 (45) |
| Lymphadenopathyc | |
| ≥50% improvement | 51/88 (58) |
| Complete resolution | 17/88 (19) |
| Splenomegaly | |
| ≥50% improvement | 27/47 (57) |
| Complete resolution | 23/47 (49) |
| Hepatomegaly | |
| ≥50% improvement | 14/24 (58) |
| Complete resolution | 11/24 (46) |
| Haemoglobin <11 g/dl at baseline to >11 g/dl post baseline | 12/49 (24) |
| Platelet counts ≤100x109/l at baseline to >50% increase or >100x109/l post baseline | 19/50 (38) |
| Neutrophils <1x109/l at baseline to >1.5x109/l | 1/17 (6) |
a Excludes patients' visits from date of first transfusion, treatment with erythropoietin, or treatment with growth factors. For patients with missing baseline data, latest screening/unscheduled data was carried forward to baseline.
b Complete resolution of constitutional symptoms (fever, night sweats, fatigue, weight loss) defined as the presence of any symptoms at baseline, followed by no symptoms present.
c Lymphadenopathy measured by sum of the products of greatest diameters (SPD) as assessed by physical examination.
Arzerra was also given to a group of patients (n=112) with bulky lymphadenopathy (defined as at least one lymph node >5 cm) who were also refractory to fludarabine. The ORR in this group was 43% (95.3% CI: 33, 53). The median progression-free survival was 5.5 months (95% CI: 4.6, 6.4) and the median overall survival was 17.4 months (95% CI: 15.0, 24.0). The response rate in patients with prior rituximab therapy was 38% (95% CI: 23, 61). These patients also experienced comparable clinical improvement, in terms of the efficacy endpoints and haematological parameters detailed above, to patients refractory to both fludarabine and alemtuzumab.
Additionally a group of patients (n=16) who were intolerant/ineligible for fludarabine treatment and/or intolerant to alemtuzumab treatment were treated with Arzerra. The overall response rate in this group was 63% (95.3% CI: 35, 85).
An open-label, two arm, randomised study (OMB114242) was conducted in patients with bulky fludarabine refractory CLL who had failed at least 2 prior therapies (n=122) comparing Arzerra monotherapy (n=79) to physicians' choice (PC) of therapy (n=43). There was no statistically significant difference in the primary endpoint of IRC assessed PFS (5.4 vs. 3.6 months, HR=0.79, p=0.27). The PFS in the monotherapy Arzerra arm was comparable to the results seen with Arzerra monotherapy in study Hx-CD20-406.
A dose-ranging study (Hx-CD20-402) was conducted in 33 patients with relapsed or refractory CLL. Patient median age was 61 years (range: 27 to 82 years), the majority were male (58%), and all were white. Treatment with Arzerra (when given as 4 once-weekly infusions), led to a 50% objective response rate in the highest dose group (1st dose: 500 mg; 2nd, 3rd and 4th dose: 2,000 mg) and included 12 partial remissions and one nodular partial remission. For the highest dose group, the median time to progression was 15.6 weeks (95% CI: 15,22.6) in the full analysis population, and 23 weeks (CI: 20,31) in responders. The duration of response was 16 weeks (CI: 13, 19) and the time to next CLL therapy was 52.4 weeks (CI: 36.9 – non-estimable).
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with Arzerra in all subsets of the paediatric population in chronic lymphocytic leukaemia (see section 4.2 for information on paediatric use).
Pharmacokinetic properties
Overall, the pharmacokinetics of ofatumumab were consistent across the indications, whether given as a single agent or in combination with fludarabine and cyclophosphamide or chlorambucil. Ofatumumab had non-linear pharmacokinetics related to its decreasing clearance over time.
Absorption
Arzerra is administered by intravenous infusion; therefore, absorption is not applicable.
Distribution
Ofatumumab has a small volume of distribution, with mean Vss values ranging from 1.7 to 8.1 l across studies, dose levels, and infusion number.
Biotransformation
Ofatumumab is a protein for which the expected metabolic pathway is degradation to small peptides and individual amino acids by ubiquitous proteolytic enzymes. Classical biotransformation studies have not been performed.
Elimination
Ofatumumab is eliminated in two ways: a target-independent route like other IgG molecules and a target-mediated route which is related to binding to B-cells. There was a rapid and sustained depletion of CD20+ B-cells after the first ofatumumab infusion, leaving a reduced number of CD20+ cells available for the antibody to bind at subsequent infusions. As a result, ofatumumab clearance values were lower and t½ values were significantly larger after later infusions than after the initial infusion; during repeated weekly infusions, ofatumumab AUC and Cmax values increased more than the expected accumulation based on first infusion data.
The main pharmacokinetic parameters of ofatumumab as a single agent or in combination are summarised in Table 5.
Table 5. Ofatumumab pharmacokinetic parameters (geometric mean):
| Population (treatment) | Dosing regimen | Cycle1 | Cmax (µg/ml) | AUC (µg.h/ml) | CL (ml/h) | t½ (days) |
|---|---|---|---|---|---|---|
| Refractory CLL (ofatumumab) | 1st infusion (300 mg) | Cycle 1 | 61.4 | |||
| 2000 mg: 8 weekly infusions followed by 4 monthly infusions | 12th dose | 827 | 166000 | 12.1 | 11.5 | |
| Previously untreated patients (ofatumumab + chlorambucil) | 1st infusion (300 mg) | Cycle 1 | 51.8 | 2620 | ||
| 1000 mg monthly infusions | Cycle 4 | 285 | 65100 | 15.4 | 18.5 | |
| Relapsed CLL (ofatumumab + FC) | 1st infusion (300 mg) | Cycle 1 | 61.4 | 3560 | ||
| 1000 mg on the 8th day of cycle 1 followed by 1000 mg monthly infusions | Cycle 4 | 313 | 89100 | 11.2 | 19.9 |
1 Cycle for which the pharmacokinetic parameters are presented in this table.
Cmax = maximum ofatumumab concentration at the end of infusion, AUC = exposure to ofatumumab over a dosing period, CL = ofatumumab clearance after multiple doses, T½ = terminal half-life
Numbers rounded to three significant digits
Special populations
Elderly (≥65 years of age)
Age was not found to be a significant factor for ofatumumab pharmacokinetics in a cross-study population pharmacokinetic analysis of patients ranging in age from 21 to 87 years of age. Paediatric population No pharmacokinetic data are available in paediatric patients.
Gender
Gender had a modest effect (12%) on ofatumumab central volume of distribution in a cross-study population analysis, with higher Cmax and AUC values observed in female patients (48% of the patients in this analysis were male and 52% were female); these effects are not considered clinically relevant, and no dose adjustment is recommended.
Renal impairment
Baseline calculated creatinine clearance was not found to be a significant factor on ofatumumab pharmacokinetics in a cross-study population analysis in patients with calculated creatinine clearance values ranging from 26 to 287 ml/min. No dose adjustment is recommended for mild to moderate renal impairment (creatinine clearance >30 ml/min). There are limited pharmacokinetic data in patients with severe renal impairment (creatinine clearance <30 ml/min).
Hepatic impairment
No formal studies were conducted to examine the effect of hepatic impairment. IgG1 molecules such as ofatumumab are catabolised by ubiquitous proteolytic enzymes, which are not restricted to hepatic tissue; therefore, changes in hepatic function are unlikely to have any effect on the elimination of ofatumumab.
Preclinical safety data
Preclinical data reveal no special hazards for humans.
Intravenous and subcutaneous administration to monkeys resulted in the expected depletion of peripheral and lymphoid tissue B-cell counts with no associated toxicological findings. As anticipated, a reduction in the IgG humoral immune response to keyhole limpet haemocyanin was noted, but there were no effects on delayed-type hypersensitivity responses. In a few animals, increased red cell destruction occurred, presumably as a result of monkey anti-drug antibodies coating the red cells. A corresponding increase in reticulocyte counts seen in these monkeys was indicative of a regenerative response in the bone marrow.
Intravenous administration of ofatumumab to pregnant cynomolgus monkeys at 100 mg/kg once weekly from days 20 to 50 of gestation did not elicit maternal or foetal toxicity or teratogenicity. At the end of organogenesis (day 48 of gestation), the ofatumumab exposure (AUCinf) corresponded to 0.46 to 3.6 times the human exposure after the eighth infusion of the maximum recommended human dose (MRHD) of 2000 mg. At day 100 of gestation, depletion of B-cells relating to the pharmacological activity of ofatumumab were observed in foetal cord blood and foetal splenic tissues. Spleen weights decreased by approximately 15% in the low-dose group and by approximately 30% in the high-dose group, compared with control values. Pre- and post-natal development studies have not been performed. Post-natal recovery has therefore not been demonstrated.
As ofatumumab is a monoclonal antibody, genotoxicity and carcinogenicity studies have not been conducted with ofatumumab.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.