AUGTYRO Capsule Ref.[107392] Active ingredients: Repotrectinib
Source: FDA, National Drug Code (US) Revision Year: 2024
Product description
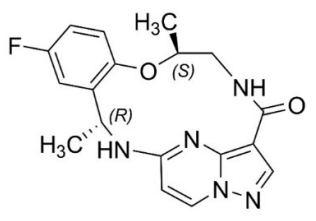
Repotrectinib is a kinase inhibitor. The molecular formula for repotrectinib is C18H18FN5O2 and the molecular weight is 355.37 Daltons. The chemical name is (3R,11S)-6-Fluoro-3,11-dimethyl10-oxa-2,13,17,18,21-pentaazatetracyclo[13.5.2.04,9.018,22]docosa-1(21),4,6,8,15(22),16,19- heptaen-14-one. The chemical structure of repotrectinib is as follows:
Repotrectinib is a white to off-white powder.
AUGTYRO (repotrectinib) capsules for oral use are supplied as printed hard shell capsules containing 40 mg of repotrectinib. Inactive ingredients are microcrystalline cellulose, sodium lauryl sulfate, croscarmellose sodium, and colloidal silicon dioxide.
The white opaque capsule shell contains gelatin and titanium dioxide. The printing ink contains shellac and FD & C blue #2 aluminum lake.
AUGTYRO (repotrectinib) capsules for oral use are supplied as printed hard shell capsules containing 160 mg of repotrectinib. Inactive ingredients are microcrystalline cellulose, sodium lauryl sulfate, croscarmellose sodium, magnesium stearate, and colloidal silicon dioxide.
The blue opaque capsule shell contains gelatin, titanium dioxide and FD & C blue #1. The printing ink contains shellac and titanium dioxide.
| Dosage Forms and Strengths |
|---|
|
Capsules: 40 mg, white, opaque, immediate release, Size 0, hard shell capsule, filled with white to off-white powder which may appear as a plug, imprinted with "REP 40" in blue text on the cap. Capsules: 160 mg, blue, opaque, immediate release, Size 0, hard shell capsule, filled with white to off-white powder which may appear as a plug, imprinted with "REP 160" in white text on the cap. |
| How Supplied |
|---|
|
AUGTYRO (repotrectinib) 40 mg, Size 0, white opaque cap, white opaque body, hard shell capsules, filled with a white to off-white powder which may appear as a plug, imprinted with "REP 40" in blue text on the cap are supplied as follows:
AUGTYRO (repotrectinib) 160 mg, Size 0, blue opaque cap, blue opaque body, hard shell capsules, filled with a white to off-white powder which may appear as a plug, imprinted with "REP 160" in white text on the cap are supplied as follows:
Distributed by: Bristol-Myers Squibb Company, Princeton, NJ 08543 USA |
Drugs
| Drug | Countries | |
|---|---|---|
| AUGTYRO | Lithuania, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.