AUSTEDO Coated tablet Ref.[10001] Active ingredients:
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
The precise mechanism by which deutetrabenazine exerts its effects in the treatment of tardive dyskinesia and chorea in patients with Huntington’s disease is unknown but is believed to be related to its effect as a reversible depletor of monoamines (such as dopamine, serotonin, norepinephrine, and histamine) from nerve terminals. The major circulating metabolites (α-dihydrotetrabenazine [HTBZ] and β-HTBZ) of deutetrabenazine, are reversible inhibitors of VMAT2, resulting in decreased uptake of monoamines into synaptic vesicles and depletion of monoamine stores.
12.2. Pharmacodynamics
Cardiac Electrophysiology
The effect of a single 12-mg or 24-mg dose of AUSTEDO on the QT interval was studied in a randomized, double-blind, placebo-controlled crossover study in healthy male and female subjects with moxifloxacin as a positive control. At 24 mg, AUSTEDO caused an approximately 4.5 msec mean increase in QTc (90% CI: 2.4, 6.5 msec). Effects at higher exposures to AUSTEDO or its metabolites have not been evaluated.
Melanin Binding
Deutetrabenazine or its metabolites bind to melanin-containing tissues (i.e., eye, skin, fur) in pigmented rats. After a single oral dose of radiolabeled deutetrabenazine, radioactivity was still detected in eye and fur at 35 days following dosing [see Warnings and Precautions (5.9)].
12.3. Pharmacokinetics
After oral dosing up to 25 mg, plasma concentrations of deutetrabenazine are generally below the limit of detection because of the extensive hepatic metabolism of deutetrabenazine to the active deuterated dihydro metabolites (HTBZ), α-HTBZ and β-HTBZ. Linear dose dependence of Cmax and AUC was observed for the active metabolites following single or multiple doses of deutetrabenazine (6 mg to 24 mg and 7.5 mg twice daily to 22.5 mg twice daily).
Absorption
Following oral administration of deutetrabenazine, the extent of absorption is at least 80%.
Plasma concentrations of deutetrabenazine are generally below the limit of detection after oral dosing. Peak plasma concentrations (Cmax) of deuterated α-HTBZ and β-HTBZ are reached within 3 to 4 hours after dosing.
Effect of Food
The effects of food on the bioavailability of AUSTEDO were studied in subjects administered a single dose with and without food. Food had no effect on the area under the plasma concentration-time curve (AUC) of α-HTBZ or β-HTBZ, although Cmax was increased by approximately 50% in the presence of food [see Dosage and Administration (2.1)].
Distribution
The median volume of distribution (Vc/F) of the α-HTBZ, and the β-HTBZ metabolites of AUSTEDO are approximately 500 L and 730 L, respectively.
Results of PET-scan studies in humans show that following intravenous injection of 11C-labeled tetrabenazine or α-HTBZ, radioactivity is rapidly distributed to the brain, with the highest binding in the striatum and lowest binding in the cortex.
The in vitro protein binding of tetrabenazine, α-HTBZ, and β-HTBZ was examined in human plasma for concentrations ranging from 50 to 200 ng/mL. Tetrabenazine binding ranged from 82% to 85%, α-HTBZ binding ranged from 60% to 68%, and β-HTBZ binding ranged from 59% to 63%.
Elimination
AUSTEDO is primarily renally eliminated in the form of metabolites.
The half-life of total (α+β)-HTBZ from deutetrabenazine is approximately 9 to 10 hours.
The median clearance values (CL/F) of the α-HTBZ, and the β-HTBZ metabolites of AUSTEDO are approximately 47 L/hour and 70 L/hour, respectively, in the Huntington’s disease patient population.
Metabolism
In vitro experiments in human liver microsomes demonstrate that deutetrabenazine is extensively biotransformed, mainly by carbonyl reductase, to its major active metabolites, α-HTBZ and β-HTBZ, which are subsequently metabolized primarily by CYP2D6, with minor contributions of CYP1A2 and CYP3A4/5, to form several minor metabolites.
Excretion
In a mass balance study in 6 healthy subjects, 75% to 86% of the deutetrabenazine dose was excreted in the urine, and fecal recovery accounted for 8% to 11% of the dose. Urinary excretion of the α-HTBZ and β-HTBZ metabolites from deutetrabenazine each accounted for less than 10% of the administered dose. Sulfate and glucuronide conjugates of the α-HTBZ and β-HTBZ metabolites of deutetrabenazine, as well as products of oxidative metabolism, accounted for the majority of metabolites in the urine.
Specific Populations
Male and Female Patients
There is no apparent effect of gender on the pharmacokinetics of α-HTBZ and β‑HTBZ of deutetrabenazine.
Patients with Renal Impairment
No clinical studies have been conducted to assess the effect of renal impairment on the PK of AUSTEDO.
Patients with Hepatic Impairment
The effect of hepatic impairment on the pharmacokinetics of deutetrabenazine and its primary metabolites has not been studied. However, in a clinical study conducted to assess the effect of hepatic impairment on the pharmacokinetics of tetrabenazine, a closely related VMAT2 inhibitor, the exposure to α-HTBZ and β-HTBZ was up to 40% greater in patients with hepatic impairment, and the mean tetrabenazine Cmax in patients with hepatic impairment was up to 190-fold higher than in healthy subjects [see Contraindications (4), Use in Specific Populations (8.6)].
Poor CYP2D6 Metabolizers
Although the pharmacokinetics of deutetrabenazine and its metabolites have not been systematically evaluated in patients who do not express the drug metabolizing enzyme CYP2D6, it is likely that the exposure to α-HTBZ and β-HTBZ would be increased similarly to taking strong CYP2D6 inhibitors (approximately 3-fold) [see Dosage and Administration (2.4), Drug Interactions (7.1)].
Drug Interaction Studies
Deutetrabenazine, α-HTBZ, and β-HTBZ have not been evaluated in in vitro studies for induction or inhibition of CYP enzymes or interaction with P-glycoprotein. The results of in vitro studies of tetrabenazine do not suggest that tetrabenazine or its α-HTBZ or β-HTBZ metabolites are likely to result in clinically significant inhibition of CYP2D6, CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2E1, or CYP3A. In vitro studies suggest that neither tetrabenazine nor its α-HTBZ or β-HTBZ metabolites are likely to result in clinically significant induction of CYP1A2, CYP3A4, CYP2B6, CYP2C8, CYP2C9, or CYP2C19. Neither tetrabenazine nor its α-HTBZ or β-HTBZ metabolites are likely to be a substrate or inhibitor of P-glycoprotein at clinically relevant concentrations in vivo.
The deutetrabenazine metabolites, 2-methylpropanoic acid of β-HTBZ (M1) and monohydroxy tetrabenazine (M4), have been evaluated in a panel of in vitro drug-drug interaction studies; the results indicate that M1/M4 are not expected to cause clinically relevant drug interactions.
CYP2D6 Inhibitors
In vitro studies indicate that the α-HTBZ and β-HTBZ metabolites of deutetrabenazine are substrates for CYP2D6. The effect of CYP2D6 inhibition on the pharmacokinetics of deutetrabenazine and its metabolites was studied in 24 healthy subjects following a single 22.5 mg dose of deutetrabenazine given after 8 days of administration of the strong CYP2D6 inhibitor paroxetine 20 mg daily. In the presence of paroxetine, systemic exposure (AUCinf) of α-HTBZ was 1.9-fold higher and β-HTBZ was 6.5-fold higher, resulting in approximately 3-fold increase in AUCinf for total (α+β)-HTBZ. Paroxetine decreased the clearance of α-HTBZ and β-HTBZ metabolites of AUSTEDO with corresponding increases in mean half-life of approximately 1.5-fold and 2.7-fold, respectively. In the presence of paroxetine, Cmax of α-HTBZ and β-HTBZ were 1.2-fold and 2.2-fold higher, respectively.
The effect of moderate or weak CYP2D6 inhibitors such as duloxetine, terbinafine, amiodarone, or sertraline on the exposure of deutetrabenazine and its metabolites has not been evaluated.
Digoxin
AUSTEDO was not evaluated for interaction with digoxin. Digoxin is a substrate for P-glycoprotein. A study in healthy subjects showed that tetrabenazine (25 mg twice daily for 3 days) did not affect the bioavailability of digoxin, suggesting that at this dose, tetrabenazine does not affect P‑glycoprotein in the intestinal tract. In vitro studies also do not suggest that tetrabenazine or its metabolites are P-glycoprotein inhibitors.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
No carcinogenicity studies were performed with deutetrabenazine.
No increase in tumors was observed in p53+/– transgenic mice treated orally with tetrabenazine at doses of 0, 5, 15, and 30 mg/kg/day for 26 weeks.
Mutagenesis
Deutetrabenazine and its deuterated α-HTBZ and β-HTBZ metabolites were negative in in vitro (bacterial reverse mutation and chromosome aberration in human peripheral blood lymphocytes) assays in the presence or absence of metabolic activation and in the in vivo micronucleus assay in mice.
Impairment of Fertility
The effects of deutetrabenazine on fertility have not been evaluated. Oral administration of deutetrabenazine (doses of 5, 10, or 30 mg/kg/day) to female rats for 3 months resulted in estrous cycle disruption at all doses; the lowest dose tested was similar to the maximum recommended human dose (48 mg/day) on a body surface area (mg/m²) basis.
Oral administration of tetrabenazine (doses of 5, 15, or 30 mg/kg/day) to female rats prior to and throughout mating, and continuing through day 7 of gestation, resulted in disrupted estrous cyclicity at doses greater than 5 mg/kg/day. No effects on mating and fertility indices or sperm parameters (motility, count, density) were observed when males were treated orally with tetrabenazine at doses of 5, 15 or 30 mg/kg/day prior to and throughout mating with untreated females.
14. Clinical Studies
14.1 Chorea Associated with Huntington’s Disease
Double-Blind, Placebo-Controlled Study
The efficacy of AUSTEDO as a treatment for chorea associated with Huntington’s disease was established primarily in Study 1, a randomized, double-blind, placebo-controlled, multi-center trial conducted in 90 ambulatory patients with manifest chorea associated with Huntington’s disease. The diagnosis of Huntington’s disease was based on family history, neurological exam, and genetic testing. Treatment duration was 12 weeks, including an 8-week dose titration period and a 4-week maintenance period, followed by a 1-week washout. Patients were not blinded to discontinuation. AUSTEDO was started at 6 mg per day and titrated upward, at weekly intervals, in 6 mg increments until satisfactory treatment of chorea was achieved, intolerable side effects occurred, or until a maximal dose of 48 mg per day was reached. The primary efficacy endpoint was the Total Maximal Chorea Score, an item of the Unified Huntington’s Disease Rating Scale (UHDRS). On this scale, chorea is rated from 0 to 4 (with 0 representing no chorea) for 7 different parts of the body. The total score ranges from 0 to 28.
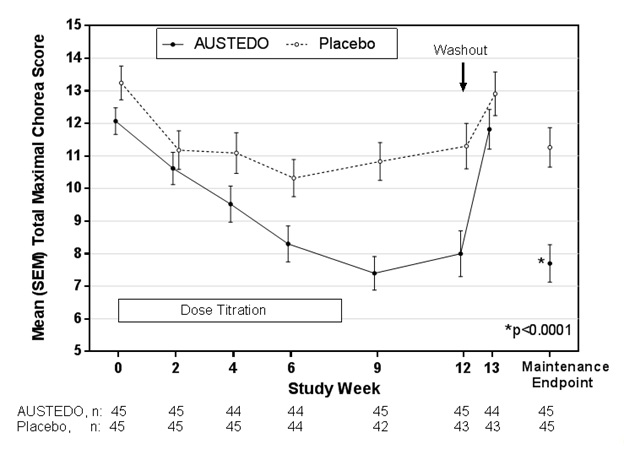
Of the 90 patients enrolled, 87 patients completed the study. The mean age was 54 (range 23 to 74). Patients were 56% male and 92% Caucasian. The mean dose after titration was 40 mg per day. Table 4 and Figure 1 summarize the effects of AUSTEDO on chorea based on the Total Maximal Chorea Score. Total Maximal Chorea Scores for patients receiving AUSTEDO improved by approximately 4.4 units from baseline to the maintenance period (average of Week 9 and Week 12), compared to approximately 1.9 units in the placebo group. The treatment effect of -2.5 units was statistically significant (p<0.0001). The Maintenance Endpoint is the mean of the Total Maximal Chorea Scores for the Week 9 and Week 12 visits. At the Week 13 follow-up visit (1 week after discontinuation of the study medication), the Total Maximal Chorea Scores of patients who had received AUSTEDO returned to baseline (Figure 1).
Table 4. Change from Baseline to Maintenance Therapy in Total Maximal Chorea (TMC)* Score in Patients with Huntington’s Disease Treated with AUSTEDO in Study 1:
| Motor Endpoint | AUSTEDO N=45 | Placebo N=45 | p value |
|---|---|---|---|
| Change in Total Chorea Score* from Baseline to Maintenance Therapy† | -4.4 | -1.9 | <0.0001 |
==*==TMC is a subscale of the Unified Huntington’s Disease Rating Scale (UHDRS)
† Primary efficacy endpoint
Figure 1. Total Maximal Chorea Score Over Time in Study 1:
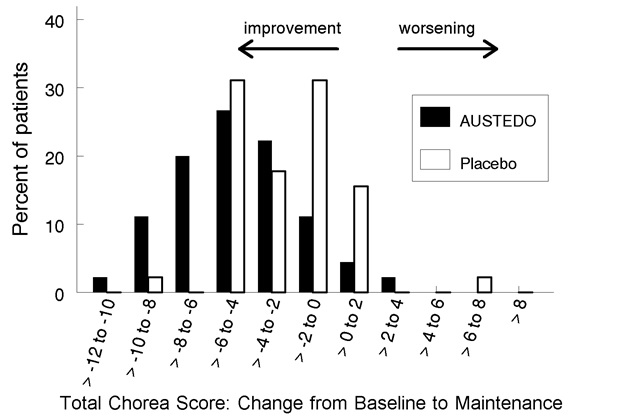
Figure 2. Distribution of the Change in Total Maximal Chorea Scores in Study 1:
Figure 2 shows the distribution of values for the change in Total Maximal Chorea Score in Study 1. Negative values indicate a reduction in chorea and positive numbers indicate an increase in chorea.
A patient-rated global impression of change assessed how patients rated their overall Huntington’s disease symptoms. Fifty-one percent of patients treated with AUSTEDO rated their symptoms as “Much Improved” or “Very Much Improved” at the end of treatment, compared to 20% of placebo-treated patients.
In a physician-rated clinical global impression of change, physicians rated 42% percent of patients treated with AUSTEDO as “Much Improved” or “Very Much Improved” at the end of treatment compared to 13% of placebo-treated patients.
14.2 Tardive Dyskinesia
The efficacy of AUSTEDO in the treatment for tardive dyskinesia was established in two 12‑week, randomized, double-blind, placebo-controlled, multi-center trials conducted in 335 adult ambulatory patients with tardive dyskinesia caused by use of dopamine receptor antagonists. Patients had a history of using a dopamine receptor antagonist (antipsychotics, metoclopramide) for at least 3 months (or 1 month in patients 60 years of age and older). Concurrent diagnoses included schizophrenia/schizoaffective disorder (62%) and mood disorder (33%). With respect to concurrent antipsychotic use, 64% of patients were receiving atypical antipsychotics, 12% were receiving typical or combination antipsychotics, and 24% were not receiving antipsychotics.
The Abnormal Involuntary Movement Scale (AIMS) was the primary efficacy measure for the assessment of tardive dyskinesia severity. The AIMS is a 12-item scale; items 1 to 7 assess the severity of involuntary movements across body regions and these items were used in this study. Each of the 7 items was scored on a 0 to 4 scale, rated as: 0=not present; 1=minimal, may be extreme normal (abnormal movements occur infrequently and/or are difficult to detect); 2=mild (abnormal movements occur infrequently and are easy to detect); 3=moderate (abnormal movements occur frequently and are easy to detect) or 4 =severe (abnormal movements occur almost continuously and/or of extreme intensity). The AIMS total score (sum of items 1 to 7) could thus range from 0 to 28, with a decrease in score indicating improvement.
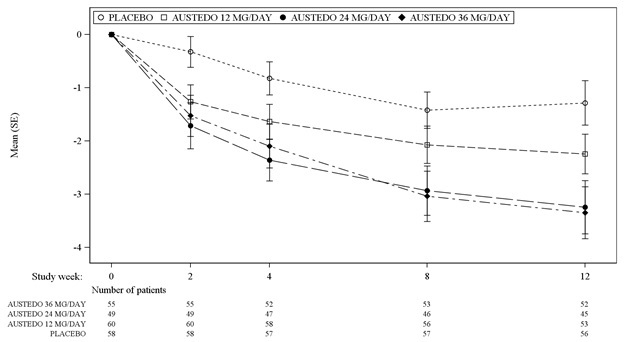
In Study 1, a 12-week, placebo-controlled, fixed-dose trial, adults with tardive dyskinesia were randomized 1:1:1:1 to 12 mg AUSTEDO, 24 mg AUSTEDO, 36 mg AUSTEDO, or placebo. Treatment duration included a 4-week dose escalation period and an 8-week maintenance period followed by a 1-week washout. The dose of AUSTEDO was started at 12 mg per day and increased at weekly intervals in 6 mg/day increments to a dose target of 12 mg, 24 mg or 36 mg per day. The population (n= 222) was 21 to 81 years old (mean 57 years), 48% male, and 79% Caucasian. In Study 1, the AIMS total score for patients receiving AUSTEDO demonstrated statistically significant improvement, from baseline to Week 12, of 3.3 and 3.2 units for the 36 mg and 24 mg arms, respectively, compared with 1.4 units in placebo (Study 1 in Table 5). The improvements on the AIMS total score over the course of the study are displayed in Figure 3. Data did not suggest substantial differences in efficacy across various demographic groups. The treatment response rate distribution, based on magnitude of AIMS total score from baseline to week 12 is displayed in Figure 4.
The mean changes in the AIMS total score by visit are shown in Figure 3.
In Study 2, a 12-week, placebo-controlled, flexible-dose trial, adults with tardive dyskinesia (n=113) received daily doses of placebo or AUSTEDO, starting at 12 mg per day with increases allowed in 6-mg increments at 1-week intervals until satisfactory control of dyskinesia was achieved, until intolerable side effects occurred, or until a maximal dose of 48 mg per day was reached. Treatment duration included a 6-week dose titration period and a 6-week maintenance period followed by a 1-week washout. The population was 25 to 75 years old (mean 55 years), 48% male, and 70% Caucasian. Patients were titrated to an optimal dose over 6 weeks. The average dose of AUSTEDO after treatment was 38.3 mg per day. There was no evidence suggesting substantial differences in efficacy across various demographic groups. In Study 2, AIMS total score for patients receiving AUSTEDO demonstrated statistically significant improvement by 3.0 units from baseline to endpoint (Week 12), compared with 1.6 units in the placebo group with a treatment effect of -1.4 units. Table 5 summarizes the effects of AUSTEDO on tardive dyskinesia based on the AIMS.
Table 5. Improvement in AIMS Total Score in Patients Treated with AUSTEDO in Study 1 and Study 2:
| Study | Treatment Group | Primary Efficacy Measure: AIMS Total Score | ||
|---|---|---|---|---|
| Mean Baseline Score (SD) | LS Mean Change from Baseline (SE) | Treatment Effect (95% CI) | ||
| Study 1 | AUSTEDO 36 mg* (n=55) | 10.1 (3.21) | -3.3 (0.42) | -1.9 (-3.09, -0.79) |
| AUSTEDO 24 mg (n=49) | 9.4 (2.93) | -3.2 (0.45) | -1.8 (-3.00, -0.63) | |
| AUSTEDO 12 mg (n=60) | 9.6 (2.40) | -2.1 (0.42) | -0.7 (-1.84, 0.42) | |
| Placebo (n=58) | 9.5 (2.71) | -1.4 (0.41) | ||
| Study 2 | AUSTEDO (12-48 mg/day)* (n=56) | 9.7 (4.14) | -3.0 (0.45) | -1.4 (-2.6, -0.2) |
| Placebo (n=57) | 9.6 (3.78) | -1.6 (0.46) | ||
* Dose that was statistically significantly different from placebo after adjusting for multiplicity.
LS Mean = Least-squares mean; SD = Standard deviation; SE = Standard error; CI = 2-sided 95% confidence interval
Figure 3. Least Square Means of Change in AIMS Total Score from Baseline for AUSTEDO Compared to Placebo (Study 1):
SE = Standard error
Figure 4. Percent of Patients with Specified Magnitude of AIMS Total Score Improvement at the End of Week 12 (Study 1):
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.