AVIGAN Tablets Ref.[8719] Active ingredients: Favipiravir
Publisher: Manufactured and Marketed by: TOYAMA CHEMICAL CO., LTD. 2-5, Nishishinjuku 3-chome, Shinjuku-ku, Tokyo, 160-0023, Japan
5.1. Pharmacodynamic properties
Mechanism of action
Favipiravir (originally known as T-705) is an orally administered novel anti-viral compound with a unique mechanism of action that is active against a wide range of RNA-based viruses in laboratory tests. Favipiravir is a viral RNA polymerase inhibitor with a new mechanism of action, inhibiting viral gene replication within infected cells to prevent the propagation. Favipiravir is phosphoribosylated by cellular enzymes to its active form, favipiravir-ribofuranosyl-5-triphosphate (RTP). Favipiravir is active against a broad range of influenza viruses, including A(H1N1)pdm09, A(H5N1) and the recently emerged A(H7N9) avian virus. It also inhibits influenza strains resistant to current antiviral drugs, and shows a synergistic effect in combination with oseltamivir, thereby expanding influenza treatment options. With regards to the activity against human DNA polymerases α, β and γ, favipiravir RTP (1000 μmol/L) showed no inhibitory effect on α, 9.1-13.5% inhibitory effect on β and 11.7-41.2% inhibitory effect on γ. Inhibitory concentration (IC50) of favipiravir RTP on human RNA polymerase II was 905 μmol/L.
In vitro antiviral activity
Favipiravir showed antiviral activity against type A and type B influenza virus laboratory strains with an EC50 of 0.014–0.55 μg/mL.
The EC50 against seasonal type A and type B influenza viruses including strains resistant to adamantane (amantadine and rimantadine), oseltamivir or zanamivir was 0.03–0.94 and 0.09–0.83 μg/mL, respectively.
The EC50 against type A influenza viruses (including strains resistant to adamantane, oseltamivir or zanamivir) such as swine-origin type A and avian-origin type A including highly-pathogenic strains (including H5N1 and H7N9) was 0.06–3.53 μg/mL.
The EC50 against type A and type B influenza viruses resistant to adamantane, oseltamivir and zanamivir was 0.09–0.47 μg/mL, and no cross resistance was observed.
Therapeutic effect in animal models
In mouse infection models inoculated with influenza viruses A (H7N9), A (H1N1) pdm09 or A (H3N2), decrease of virus titers in lung tissues was observed by a 5-day oral administration of favipiravir with a dose of ≤60 mg/kg/day.
In mouse infection models inoculated with influenza viruses A (H3N2) or A (H5N1), therapeutic effect was observed by a 5-day oral administration of favipiravir with a dose of 30 mg/kg/day.
In a SCID mouse infection model inoculated with an influenza virus A (H3N2), therapeutic effect was observed by a 14-day oral administration of favipiravir with a dose of 30 mg/kg/day.
Resistance
No change of susceptibility of type A influenza viruses to favipiravir was observed after 30 passages in the presence of favipiravir, and no resistant viruses have been selected. In clinical studies including the global phase III study, information about emergence of favipiravir-resistant influenza viruses has not been obtained.
Clinical studies
Results in non-Japanese
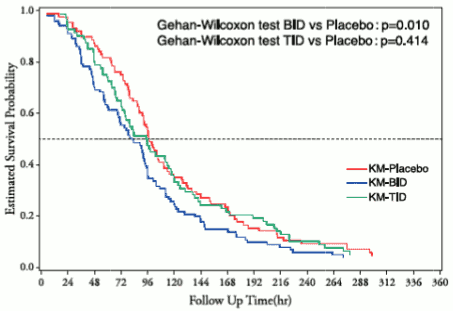
A placebo-controlled phase I/II study in type A or o type B influenza patients was conducted (1800 mg/800 mg BID, oral administration mg of favipiravir 1800 mg twice daily for 1 day followed by 800 mg twice daily for 4 days; 2400 mg/600 mg TID, oral administration of favipiravir 2400mg + 600 mg + 600 mg for 1 day followed by 600 mg three times daily for 4 days)Note14. With regards to the primary endpointNote 15, favipiravir 1800 mg/800 mg BID (101 patients) demonstrated significant difference in time to alleviation of influenza symptoms compared to placebo (88 patients) (p=0.01, Gehan-Wilcoxon test), but favipiravir 2400 mg/600 mg TID (82 patients) failed to demonstrate significant difference (p=0.414, Gehan-Wilcoxon test).
Figure 1. Time to alleviation of influenza symptoms:
Note 14: The approved dosage of favipiravir is "1600 mg orally twice daily for 1 day followed by 600 mg orally twice daily for 4 days".
Note 15: Time required to alleviate 6 primary influenza symptoms (cough, sore throat, headache, nasal congestion, body aches and pains, fatigue [tiredness]) and body temperature.
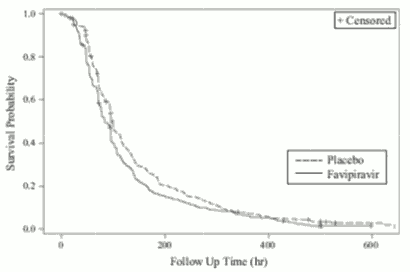
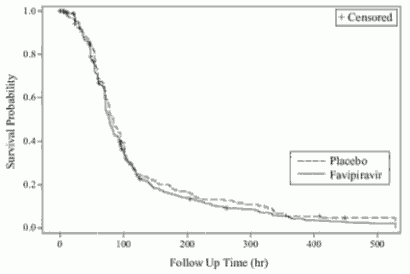
Two placebo-controlled phase III studies in type A or type B mg influenza patients (oral administration of favipiravir 1800 mg twice daily for 1 day followed by 800 mg twice daily for 4 days [1800 mg/800 mg BID])Note16 with the primary endpoint: the time required to alleviate primary influenza symptomsNote17, were conducted (Study 1 and Stuudy 2). The results are as follows.
Results of primary analysis (ITTI population):
Figure 2. Kaplan-Meier Plot with regard to primary endpointNote17 (ITTI population, Study 1):
Figure 3. Kaplan-Meier Plot with regard to primary endpointNote17 (ITTI population, Study 2):
Note 16: The approved dosage of favipiravir is "1600 mg orally twice daily for 1 day followed by 600 mg orally twice daily for 4 days"
Note 17: Time required to alleviate 6 primary influenza symptoms (cough, sore throat, headache, nasal congestion, body aches and pains, fatigue [tiredness]) and resolution of fever. Alleviation was defined as all of the 6 influenza symptoms had been either absent or mild and fever had resolved, with both maintained for at least 21.5 hours.
Note 18: Peto-Peto-Prentice test
Reference: Global phase III clinical study (adults)
A global phase III clinical study of favipiravir (the dosageNote19 was different from the approved dosage for adults) versus oseltamivir phosphate (75 mg twice daily for 5 days) was conducted in patients with type A or type B influenza (640 patients [467 patients in Japan, 55 patients in Korea, and 118 patients in Taiwan]). The median time (95% CI) to alleviation of primary influenza symptomsNote20 was 63.1 hours (55.5, 70.4) for favipiravir group (377 patients) and 51.2 hours (45.9, 57.6) for oseltamivir phosphate group (380 patients). The hazard ratio (95% CI) of favipiravir to oseltamivir phosphate for time to alleviation of primary influenza symptoms was 0.818 (0.707, 0.948), and the efficacy of favipiravir was not demonstrated (p=0.007, log-rank test).
Note 19: 1200 mg + 400 mg on Day 1 followed by 400 mg twice daily for 4 days. The approved dosage of favipiravir is "1600 mg for 4 day orally twice daily for 1 day followed by 600 mg orally twice daily for 4 days".
Note 20: Time required for 7 primary influenza symptoms (cough, sore throat, headache, nasal congestion, feeling feverish, body pains, fatigue [tiredness]) to alleviate after the start of study drug administration (the time point when all symptoms were scored 1 or below). "Alleviation" was defined as the state graded by the investigator based on the record of the patient diary remain unchanged for 21.5 hours or longer after all of the scores decrease to 1 or below.
Reference: Phase II clinical study in non-Japanese (adults)
A placebo-controlled phase II study of favipiravir was conducted in patients with type A or type B influenza (1000 mg/400 mg BID, oral administration of favipiravir 1000 mg twice daily for 1 day followed by 400 mg twice daily for 4 days; 1200 mg/800 mg BID, oral administration of favipiravir 1200 mg twice daily for 1 day followed by 800 mg twice daily for 4 days; placebo, twice daily)Note20. The median time (95% CI) to alleviation of primary influenza symptomsNote21 was 100.4 hours (82.4, 119.8) for 1000 mg/400 mg BID group (88 patients), 86.5 hours (79.2, 102.1) for 1200 mg/800 mg BID group (121 patients), and 91.9 hours (70.3, 105.4) for placebo group (124 patients). There was no statistically significant difference between either favipiravir group and placebo group (p>0.05, Gehan-Wilcoxon test; A step-down approach was used to regulate the overall type I error rate for the multiple comparisons).
Note 19: The approved dosage of favipiravir is "1600 mg orally twice daily for 1 day followed by 600 mg orally twice daily for 4 days".
Note 20: Time required to "alleviate" 6 primary influenza symptoms (cough, sore throat, headache, nasal congestion, body aches and pains, fatigue [tiredness]) and body temperature, where alleviation was defined as the state where all of the scores and temperature remain unchanged for 21.5 hours or longer after all of the scores decrease to 1 or below and temperature returned to less than 38.0°C for 20 to <65 years old and less than 37.8°C for patients ≥65 years old.
5.2. Pharmacokinetic properties
Blood Concentrations
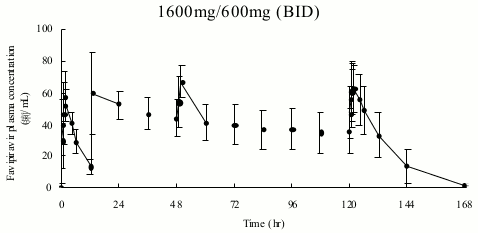
The following table shows pharmacokinetic parameters of favipiravir after an oral administration in 8 healthy adults at 1600 mg twice daily for 1 day, then 600 mg twice daily for 4 days followed by 600 mg once daily for 1 day (1600 mg/600 mg BID).
Pharmacokinetic parameters of favipiravir:
| Dosage | CmaxNote2 (μg/mL) | AUCNote2,3 (μg·hr/mL) | TmaxNote4 (hr) | T1/2Note5 (hr) | |
|---|---|---|---|---|---|
| 1600 mg/600 mg BID | Day 1 | 64.56 (17.2) | 446.09 (28.1) | 1.5 (0.75, 4) | 4.8±1.1 |
| Day 6 | 64.69 (24.1) | 553.98 (31.2) | 1.5 (0.75, 2) | 5.6±2.3 |
Note 2: Geometric mean (CV%)
Note 3: Day 1: AUC0-∞, Day 6: AUCτ
Note 4: Median (minimum, maximum)
Note 5: Mean±SD
Figure 4. Time course of plasma concentration of favipiravir (mean±SD):
Following multiple oral administration of favipiravir for 7 daysNote6 to an healthy adult who appeared to have little AO activity, the estimated AUC of unchanged drug was 1452.73 μg·hr/mL on Day 1 and 1324.09 μg·hr/mL on Day 7.
Note 6: 1200 mg + 400 mg on Day 1, then 400 mg twice daily on Days 2 to 6 followed by 400 mg once daily on Day 7. The approved dosage of favipiravir is "1600 mg orally twice daily for 1 day followed by 600 mg orally twice daily for 4 days".
Distribution
When favipiravir was orally administered to 20 healthy adult male subjects at 1200 mg twice daily for 1 day followed by 800 mg twice daily for 4 days (1200 mg/800 mg BID)Note7, the geometric mean concentration of the drug in semen was 18.341 μg/mL on Day 3, and 0.053 μg/mL on the second day after the treatment. The semen levels became below the limit of quantification (0.02 μg/mL) in all subjects in 7 days after the end of the treatment. The mean ratio of the drug concentration in semen to that in plasma was 0.53 on Day 3 and 0.45 on the second day after the treatment.
The serum protein binding ratio was 53.4 to 54.4% (in vitro, centrifugal ultrafiltration) at 0.3 to 30 μg/mL.
Note 7: The approved dosage of favipiravir is "1600mg orally twice daily for 1 day followed by 600 mg orally twice daily for 4 days".
Reference: Animal data
When a single dose of 14C-favipiravir was orally administered to monkeys, it was distributed broadly in tissues. Radioactivity of each tissue peaked in 0.5 hours after the administration and changed in parallel with the radioactivity in plasma. The ratio of radioactivity in lung tissues to that in plasma was 0.51 in 0.5 hours after the administration, and the drug was distributed rapidly to respiratory tissues which were considered infection site. Radioactivity in kidney was higher than that in plasma, with a ratio of 2.66. Radioactivity in each tissue, except bones, decreased to ≤2.8% of the peak within 24 hours after the administration.
Metabolism
Favipiravir was not metabolized by cytochrome P-450 (CYP), mostly metabolized by aldehyde oxidase (AO), and partly metabolized to a hydroxylated form by xanthine oxidase (XO). In studies using human liver microsomes, formation of the hydroxylate ranged from 3.98 to 47.6 pmol/mg protein/min, with an inter-individual variation of AO activity by 12 times at maximum. A glucuronate conjugate was observed in human plasma and urine as a metabolite other than the hydroxylated form.
Excretion
Favipiravir was mainly excreted as a hydroxylated form into the urine, and little amount unchanged drug was observed. In an oral 7 day multiple dose studyNote8 with 6 healthy adults, cumulative urinary excretion ratio of the unchanged drug and the hydroxylated form was 0.8% and 53.1%, respectively, during 48 hours after the last administration.
Note 8: 1200 mg + 400 mg on Day 1, then 400 mg twice daily on Days 2 to 6 followed by 400 mg once daily on Day 7. The approved dosage of favipiravir is "1600 mg orally twice daily for 1 day followed by 600 mg orally twice daily for 4 days".
Patients with liver function impairment
When favipiravir was orally administered to subjects with mild and moderate liver function impairment (Child-Pugh classification A and B, 6 subjects each) at 1200 mg twice daily for 1 day followed by 800 mg twice daily for 4 days (1200 mg/800 mg BID)Note9, compaired to healthy adult subjects, Cmax and AUC at day 5 were approximately 1.6 fold and 1.7 fold, respectively in subjects with mild liver function impairment, and 1.4 fold and 1.8 fold, respectively in subjects with moderate liver function impairment. When favipiravir was orally administered to subjects with severe liver function impairment (Child-Pugh classification C, 4 subjects) at 800 mg twice daily for 1 day followed by 400 mg twice daily for 2 days (800 mg/400 mg BID)Note9, compaired to healthy adult subjects, Cmax and AUC at day 3 were approximately 2.1 fold and 6.3 fold, respectively.
Note 9: The approved dosage of favipiravir is "1600mg orally twice daily for 1 day followed by 600 mg orally twice daily for 4 days".
Drug Interactions
In vitro
Favipiravir inhibited irreversibly AO in a dose and time dependent manner, and inhibited CYP2C8 in a dose dependent manner. There were no inhibitory activity to XO, and weak inhibitory activity to CYP1A2, 2C9, 2C19, 2D6, 2E1 and 3A4. The hydroxylated metabolite showed weak inhibitory activity to CYP1A2, 2C8, 2C9, 2C19, 2D6, 2E1 and 3A4. Inductive effect of favipiravir on CYP was not observed.
Drug-drug Interaction Clinical Studies
Effects of co-administered drugs on pharmacokinetics of favipiravir:
| Co-administrated drug and dosage | Favipiravir dosage | n | Time of dosing | Parameter ratio for favipiravir (90% CI) (Co-administered/single administered) | |
|---|---|---|---|---|---|
| Cmax | AUC | ||||
| Theophylline 200mg twice daily on Days 1 to 9, 200mg once daily on Day 10 | 600mg twice daily on Day 6, 600mg once daily on Days 7 to 10 | 10 | Day 6 | 1.33 [1.19, 1.48] | 1.27 [1.15, 1.40] |
| Day 7 | 1.03 [0.92, 1.15] | 1.17 [1.04, 1.31] | |||
| Oseltamivir 75mg twice daily on Days 1 to 5, 75mg once daily on Day 6 | 600mg twice daily on Day 5, 600mg once daily on Day 6 | 10 | Day 6 | 0.98 [0.87, 1.10] | 1.01 [0.91, 1.11] |
| Raloxifene 60mg once daily on Days 1 to 3Note10 | 1200mg twice daily on Day 1, 800mg twice daily on Day 2, 800mg once daily on Day 3 | 17 | Day 1 | 1.00 [0.90, 1.10] | 1.03 [0.95, 1.12] |
| Day 3 | 0.90 [0.81, 0.99] | 0.85 [0.79, 0.93] | |||
| Hydralazine 5mg once daily on Day 1 and Day 5 | 1200mg/400mg on Day 1, 400mg twice daily on Days 2 to 4, 400mg once daily on Day 5 | 14 | Day 1 | 0.99 [0.92, 1.06] | 0.99 [0.92, 1.07] |
| Day 3 | 0.90 [0.81, 0.99] | 0.85 [0.79, 0.93] | |||
| Hydralazine 5mg once daily on Day 1 and Day 5 | 1200mg/400mg on Day 1, 400mg twice daily on Days 2 to 4, 400mg once daily on Day 5 | 14 | Day 1 | 0.99 [0.92, 1.06] | 0.99 [0.92, 1.07] |
| Day 5 | 0.96 [0.89, 1.04] | 1.04 [0.96, 1.12] | |||
Note 10: Results in non-Japanese
Effects of favipiravir on pharmacokinetics of co-administered drugs:
| Co-administrated drug and dosage | Favipiravir dosage | n | Time of dosing | Parameter ratio for co-administered drug [90% CI] (Co-administered/single administered) | |
|---|---|---|---|---|---|
| Cmax | AUC | ||||
| Theophylline 200mg twice daily on Days 1 to 9, 200mg once daily on Day 10 | 600mg twice daily on Day 6, 600mg once daily on Days 7 to 10 | 10 | Day 7 | 0.93 [0.85, 1.01] | 0.92 [0.87, 0.97] |
| Day 10 | 0.99 [0.94, 1.04] | 0.97 [0.91, 1.03] | |||
| Oseltamivir 75mg twice daily on Days 1 to 5, 75mg once daily on Day 6 | 600mg twice daily on Day 5, 600mg once daily on Day 6 | 10 | Day 6 | 1.10 [1.06, 1.15] | 1.14 [1.10, 1.18] |
| Acetaminophen 650mg once daily on Day 1 and Day 5Note11 | 1200mg twice daily on Day 1, 800mg twice daily on Days 2 to 4, 800mg once daily on Day 5 | 28 | Day 1 | 1.03 [0.93, 1.14] | 1.16 [1.08, 1.25] |
| Day 5 | 1.08 [0.96, 1.22] | 1.14 [1.04, 1.26] | |||
| No orethindrone/Ethhinylestradiol commbination 1mmg/0.035mg once daily on Days 1 to Day 5Note11 | 1200mg twice daily on Day 1, 800mg twice daily on Days 2 to 4, 800mg once daily on Day 5 | 25 | Day 12Note12 | 1.23 [1.16, 1.30] | 1.47 [1.42, 1.52] |
| Day 12Note13 | 1.48 [1.42, 1.54] | 1.43 [1.39, 1 1.47] | |||
| Repaglinide 0.5 once daily on Day 13Note11 | 1200mg twice daily on Day 1, 800mg twice daily on Days 2 to 4, 800mg once daily on Day 5 | 17 | Day 13 | 1.28 [1.16, 1.41] | 1.52 [1.37, 1.68] |
| Hydralazine 5mg once daily on Day 1 and Day 5 | 1200mg/400mg on Day 1, 400mg twice daily on Days 2 to 4, 400mg once daily on Day 5 | 14 | Day 1 | 0.73 [0.67, 0.81] | 0.87 [0.78, 0.97] |
| Day 5 | 0.79 [0.71, 0.88] | 0.91 [0.82, 1.01] | |||
Note 11: Results in non-Japanese
Note 12: Norethindrone
Note 13: Ethinylestradiol
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.