AZULFIDINE Tablet Ref.[49800] Active ingredients: Sulfasalazine
Source: FDA, National Drug Code (US) Revision Year: 2009
Product description
AZULFIDINE Tablets contain sulfasalazine, 500 mg, for oral administration.
Therapeutic Classification: Anti-inflammatory agent.
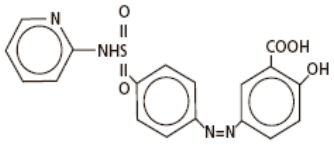
Chemical Designation: 5-([p-(2-pyridylsulfamoyl)phenyl]azo) salicylic acid.
Chemical Structure:
Molecular Formula: C18H14N4O5S
| How Supplied |
|---|
|
AZULFIDINE Tablets, 500 mg, are round, gold-colored, scored tablets, monogrammed "101" on one side and "KPh" on the other. They are available in the following package sizes: Bottles of 100 NDC 0013-0101-01 Sulfasalazine is also available as AZULFIDINE EN-tabs brand of sulfasalazine delayed release tablets, USP, 500 mg, in the following package sizes: Bottles of 100 NDC 0013-0102-01 |
Drugs
| Drug | Countries | |
|---|---|---|
| AZULFIDINE | Germany, Japan, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.