AZZALURE Powder for solution for injection Ref.[27680] Active ingredients: Botulinum toxin type A
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2021 Publisher: Ipsen Limited, 190 Bath Road, Slough, SL1 3XE, United Kingdom
4.1. Therapeutic indications
Azzalure is indicated for the temporary improvement in the appearance of moderate to severe
- Glabellar lines (vertical lines between the eyebrows) seen at maximum frown and/or
- Lateral canthal lines (crow’s feet lines) seen at maximum smile
in adult patients under 65 years, when the severity of these lines has an important psychological impact on the patient.
4.2. Posology and method of administration
Posology
Botulinum toxin units are different depending on the medicinal products. The Speywood units of Azzalure are specific to the preparation and are not interchangeable with other preparations of botulinum toxin.
Paediatric population
The safety and efficacy of Azzalure in individuals aged up to 18 years have not been established. The use of Azzalure is not recommended in subjects under 18 years.
Method of administration
Azzalure should only be administered by physicians with appropriate qualifications and expertise in this treatment and having the required equipment.
Once reconstituted, Azzalure should only be used to treat a single patient, during a single session.
For instructions on reconstitution of the medicinal product before administration, see section 6.6.
Remove any make-up and disinfect the skin with a local antiseptic.
Intramuscular injections should be performed using a sterile suitable gauge needle.
The treatment interval depends on the individual patient’s response after assessment. Treatment interval with Azzalure should not be more frequent than every three months.
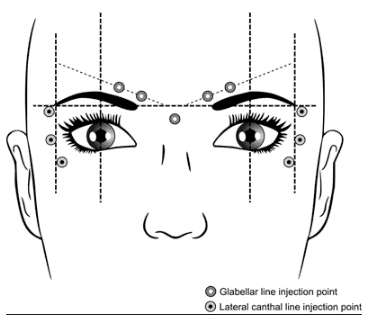
The recommended injection points for glabellar lines and lateral canthal lines are described below:
Glabellar lines
The recommended dose is 50 Speywood units of Azzalure to be divided into 5 injection sites, 10 Speywood units are to be administered intramuscularly, at right angles to the skin, into each of the 5 sites: 2 injections into each corrugator muscle and one into the procerus muscle near the nasofrontal angle as shown above.
The anatomical landmarks can be more readily identified if observed and palpated at maximal frown. Before injection, place the thumb or index finger firmly below the orbital rim in order to prevent extravasation below the orbital rim. The needle should be pointed upward and medially during the injection. In order to reduce the risk of ptosis, avoid injections near the levator palpebrae superioris muscle, particularly in patients with larger brow-depressor complexes (depressor supercilii). Injections in the corrugator muscle must be made into the central part of that muscle, at least 1 cm above the orbital rim.
In clinical studies, an optimal effect in glabellar lines was demonstrated for up to 4 months after injection. Some patients were still responders at 5 months (see section 5.1).
Lateral Canthal lines
The recommended dose per side is 30 Speywood units of Azzalure, to be divided into 3 injection sites; 10 Speywood units are to be administered intramuscularly into each injection point. Injection should be lateral (20-30° angle) to the skin and very superficial. All injection points should be at the external part of the orbicularis oculi muscle and sufficiently far from the orbital rim (approximately 1-2 cm) as shown above.
The anatomical landmarks can be more readily identified if observed and palpated at maximal smile. Care must be taken to avoid injecting the zygomaticus major/minor muscles to avoid lateral mouth drop and asymmetrical smile.
General information
In the event of treatment failure or diminished effect following repeat injections, alternative treatment methods should be employed. In case of treatment failure after the first treatment session, the following approaches may be considered:
- Analysis of the causes of failure, e.g. incorrect muscles injected, inappropriate injection technique, and formation of toxin-neutralising antibodies
- Re-evaluation of the relevance of treatment with botulinum toxin A.
The efficacy and safety of repeat injections of Azzalure has been evaluated in Glabellar lines up to 24 months and up to 8 repeat treatment cycles and for Lateral Canthal lines up to 12 months and up to 5 repeat treatment cycles.
4.9. Overdose
Excessive doses of botulinum toxin may be expected to produce neuromuscular weakness with a variety of symptoms. Respiratory support may be required where excessive doses cause paralysis of respiratory muscles. In the event of overdose the patient should be medically monitored for symptoms of excessive muscle weakness or muscle paralysis. Symptomatic treatment should be instigated if necessary.
Symptoms of overdose may not present immediately following injection.
Admission to hospital should be considered in patients presenting symptoms of botulinum toxin A poisoning (e.g. a combination of muscle weakness, ptosis, diplopia, swallowing and speech disorders, or paresis of the respiratory muscles).
6.3. Shelf life
2 years.
Reconstituted solution:
Chemical and physical in-use stability has been demonstrated for 24 hours between 2-8°C.
From a microbiological point of view, unless the method of reconstituting precludes the risks of microbial contamination, the product should be used immediately.
If not used immediately, in-use storage times and conditions are the responsibility of the user.
6.4. Special precautions for storage
Store in a refrigerator (2°C-8°C).
Do not freeze.
For storage of the reconstituted medicinal product, see section 6.3.
6.5. Nature and contents of container
125 Speywood units in a powder in a vial (Type I glass), with a stopper (halobutyl) and seal (aluminium).
Pack size of 1 or 2 vial(s).
Not all pack sizes may be marketed.
6.6. Special precautions for disposal and other handling
The instructions for use, handling and disposal should be strictly followed.
Reconstitution should be performed in accordance with good practice rules, particularly in the respect of asepsis.
Azzalure has to be reconstituted with a sodium chloride 9 mg/ml (0.9%) solution for injection.
As per the dilution table below, the requested amount of sodium chloride 9 mg/ml (0.9%) solution for injection has to be drawn up into a syringe in order to obtain a reconstituted clear solution at the following concentration:
| Amount of solvent added (0.9% sodium chloride solution) to a 125 U vial | Resulting dose |
| 0.63 ml | 10 U per 0.05 ml |
| 1.25 ml | 10 U per 0.1 ml |
The accurate measurement of 0.63 ml or 1.25 ml can be achieved using syringes graduated in 0.1 ml and 0.01 ml increments.
RECOMMENDATIONS FOR THE DISPOSAL OF CONTAMINATED MATERIALS
Immediately after use and prior to disposal, unused reconstituted Azzalure (in the vial or in the syringe) should be inactivated with 2 ml of dilute sodium hypochlorite solution at 0.55 or 1% (Dakin’s solution).
Used vials, syringes and materials should not be emptied and must be discarded into appropriate containers and disposed of in accordance with local requirements.
RECOMMENDATIONS SHOULD ANY INCIDENT OCCUR DURING THE HANDLING OF BOTULINUM TOXIN
- Any spills of the product must be wiped up: either using absorbent material impregnated with a solution of sodium hypochlorite (bleach) in case of the powder, or with dry, absorbent material in case of reconstituted product.
- The contaminated surfaces should be cleaned using absorbent material impregnated with a solution of sodium hypochlorite (bleach), then dried.
- If a vial is broken, proceed as mentioned above by carefully collecting the pieces of broken glass and wiping up the product, avoiding any cuts to the skin.
- If the product comes into contact with the skin, wash the affected area with a solution of sodium hypochlorite (bleach) then rinse abundantly with water.
- If product enters into contact with the eyes, rinse thoroughly with plenty of water or with an ophthalmic eyewash solution.
- If product enters into contact with a wound, cut or broken skin, rinse thoroughly with plenty of water and take the appropriate medical steps according to the dose injected.
These instructions for use handling and disposal should be strictly followed.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.