BAXDELA Tablet / Powder for solution for injection Ref.[9994] Active ingredients: Delafloxacin
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
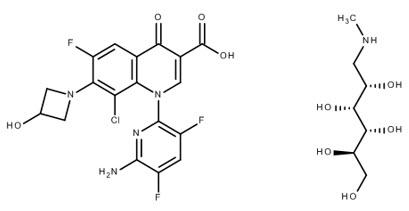
BAXDELA (delafloxacin) for Injection and BAXDELA (delafloxacin) Tablets contain meglumine salt of delafloxacin, a fluoroquinolone antibacterial. Delafloxacin meglumine is identified chemically as 1-Deoxy-1-(methylamino)-D-glucitol, 1-(6-amino-3,5-difluoropyridin-2-yl)-8-chloro-6-fluoro-7-(3-hydroxyazetidin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylate (salt), the chemical structure of which is shown below. The meglumine salt has a molecular weight of 635.97 g/mol, whereas the molecular weight of the delafloxacin free acid is 440.76 g/mol.
Figure 1. Chemical Structure:
BAXDELA is intended for intravenous infusion or oral administration. BAXDELA is supplied as a sterile, lyophilized powder for injection and oral tablets as follows:
BAXDELA for Injection
Each vial of BAXDELA for Injection, 300 mg, is a sterile lyophilized powder that contains 300 mg delafloxacin (equivalent to 433 mg delafloxacin meglumine) and the following inactive ingredients: Edetate disodium (EDTA), (3.4 mg); meglumine (59 mg); sulfobutylether-β-cyclodextrin (2400 mg).
BAXDELA Tablets
Each BAXDELA tablet for oral use contains 450 mg delafloxacin (equivalent to 649 mg delafloxacin meglumine) and the following inactive ingredients: Citric acid anhydrous (5.5 mg); crospovidone (109 mg); magnesium stearate (10 mg); microcrystalline cellulose (417 mg); povidone (34 mg); sodium bicarbonate (140 mg); sodium phosphate monobasic monohydrate (5.5 mg).
| Dosage Forms and Strengths |
|---|
|
BAXDELA for Injection: A sterile, lyophilized powder containing 300 mg delafloxacin (equivalent to 433 mg delafloxacin meglumine) in a single-dose vial, which must be reconstituted and further diluted prior to intravenous infusion. The lyophilized powder is a light yellow to tan cake, which may exhibit cracking and shrinkage and slight variation in texture and color. BAXDELA Tablets: Modified capsule shaped tablets in beige to mottled beige color with RX3341 debossed on one side containing 450 mg delafloxacin (equivalent to 649 mg delafloxacin meglumine). |
| How Supplied |
|---|
BAXDELA for InjectionBAXDELA is supplied as a sterile, lyophilized powder in single-dose clear glass vials of 300 mg delafloxacin (equivalent to 433 mg delafloxacin meglumine). The lyophilized powder is a light yellow to tan cake, which may exhibit cracking and shrinkage and slight variation in texture and color. They are supplied as follows: 300 mg single-dose vials (NDC 70842-102-01), packaged in cartons of 10 vials (NDC 70842-102-03). BAXDELA TabletsBAXDELA Tablets contain 450 mg delafloxacin (equivalent to 649 mg delafloxacin meglumine); each modified capsule-shaped tablet in beige to mottled beige color is debossed with RX3341 on one side. They are supplied as follows: Bottles of 20 tablets with child-resistant closure (NDC 70842-101-01) Unit dose blister packs which contain 20 tablets (2 blister cards of 10 tablets each) (20 tablet blister pack: NDC 70842-101-02, 10 tablet blister card: NDC 70842-101-03) |
Drugs
| Drug | Countries | |
|---|---|---|
| BAXDELA | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.