BELKYRA Solution for injection Ref.[7699] Active ingredients: Deoxycholic acid
Source: Health Products Regulatory Authority (IE) Revision Year: 2019 Publisher: Allergan Pharmaceuticals International Limited, Clonshaugh Business & Technology park, Dublin 17, D17 E400, Ireland
Therapeutic indications
Belkyra is indicated for the treatment of moderate to severe convexity or fullness associated with submental fat in adults when the presence of submental fat has an important psychological impact for the patient.
Posology and method of administration
Posology
The total volume injected and the number of treatment sessions should be tailored to the individual patient's submental fat distribution and treatment goals.
Inject 0.2 ml (2 mg) per injection site, 1 cm apart. The maximum dose of 10 ml (100 mg equivalent to 50 injections) should not be exceeded in one treatment session.
Up to a maximum of 6 treatment sessions can be performed. Most patients experience improvement in 2 to 4 treatment sessions.
The time interval between treatment sessions should be at least 4 weeks.
To improve patient comfort during injection, oral analgesics or NSAIDs, topical and/or injectable local anaesthesia (eg, lidocaine) and/or cooling using ice gel packs may be applied to the area of injection at the discretion of the healthcare professional.
Special populations
Renal impairment
No dose adjustment is considered necessary (see section 5.2).
Hepatic impairment
No dose adjustment is considered necessary (see section 5.2).
Elderly (aged 65 years and above)
No dose adjustment is considered necessary. Caution should be exercised in elderly patients (see section 4.4).
Paediatric population
There is no relevant use of Belkyra in children or adolescents.
Method of administration
The product is indicated for subcutaneous administration only.
Belkyra should only be administered by physicians with appropriate qualifications, expertise in the treatment and knowledge of the submental anatomy. Where national guidance permits, Belkyra may be administered by appropriately qualified healthcare professionals, under the supervision of a physician. Safe and effective use of Belkyra depends on appropriate patient selection, which includes knowledge of patient history of prior interventions and their potential to alter the superficial cervical anatomy.
Careful consideration should be given to the use of Belkyra in patients with excessive skin laxity, prominent platysmal bands or other conditions for which reduction of submental fat may result in an undesirable outcome.
Belkyra must be used only for one session of injection(s) per patient and the excess of unused product must be properly disposed of.
Belkyra is supplied in ready-to-use, single-use vials. Gently invert the vial several times prior to use. Do not dilute.
Insert the needle perpendicular to the skin for injections with Belkyra.
Needle placement with respect to the mandible is very important as it reduces the potential for injury to the marginal mandibular nerve, a motor branch of the facial nerve. Injury to the nerve presents as an asymmetrical smile due to paresis of lip depressor muscles.
To avoid injury to the marginal mandibular nerve:
- Do not inject above the inferior border of the mandible.
- Do not inject within a region defined by a 1-1.5 cm line below the inferior border (from the angle of the mandible to the mentum).
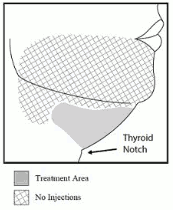
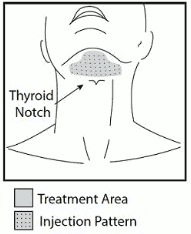
- Inject Belkyra only within the target submental fat treatment area (see Figures 1 and 3).
Figure 1. Avoid the Marginal Mandibular Nerve Area:
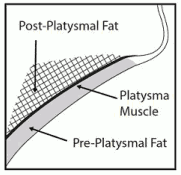
Avoid injection into the platysma. Prior to each treatment session, palpate the submental area to ensure sufficient submental fat and to identify subcutaneous fat between the dermis and platysma (pre-platysmal fat) within the target treatment area (Figure 2).
Figure 2. Sagittal View of Platysma Area:
Outline the planned treatment area with a surgical pen and apply a 1 cm² injection grid to mark the injection sites (Figures 2 and 3).
Figure 3. Treatment Area and Injection Pattern:
Do not inject Belkyra outside the defined parameters.
The solution for injection should be inspected visually prior to use. Only clear, colourless solutions free of visible particles should be used.
Overdose
No overdosing with Belkyra in humans has been reported.
Injection of increased volume or decreasing the spacing between injections of Belkyra may be expected to increase risk of local adverse effects. Non-treatment area or systemic adverse reactions were infrequent during clinical studies of doses up to 200 mg.
Shelf life
30 months.
The product should be used immediately once the vial stopper has been penetrated.
If not used immediately, in-use storage times and conditions are the responsibility of the user.
Special precautions for storage
This medicinal product does not require any special storage conditions.
For storage conditions after first opening of the medicinal product, see section 6.3.
Nature and contents of container
Solution for injection in a vial (Type I glass), fitted with a stopper (chlorobutyl rubber) and a seal (aluminium) with flip-top lid (polypropylene).
One carton contains 4 vials. Each vial contains 2 ml solution for injection.
Special precautions for disposal and other handling
Each vial is for single patient use only. After use, discard any unused product.
Belkyra shall be prepared for injections in the following way:
- Remove the flip-off cap from the vial and clean the penetrable stopper of the vial with an antiseptic. If the vial, seal, or flip-off cap is damaged, do not use.
- Attach a large bore sterile needle to a sterile single-use 1 ml syringe.
- Introduce the large bore sterile needle into the stopper of the vial and draw 1 ml of Belkyra into the 1 ml syringe.
- Replace the large bore needle with a 30 gauge (or smaller) 0.5-inch needle. Expel any air bubbles in the syringe barrel before injecting the product into the subcutaneous fat.
- To withdraw remaining contents of the vial, repeat steps 3 and 4.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.