BEOVU Solution for injection Ref.[9915] Active ingredients: Brolucizumab
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: Novartis Europharm Limited, Vista Building, Elm Park, Merrion Road, Dublin 4, Ireland
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Ophthalmologicals, antineovascularisation agents
ATC code: S01LA06
Mechanism of action
Brolucizumab is a humanised monoclonal single chain Fv (scFv) antibody fragment with a molecular weight of ~26 kDa.
Increased levels of signalling through the vascular endothelial growth factor A (VEGF-A) pathway are associated with pathological ocular angiogenesis and retinal oedema. Brolucizumab binds with high affinity to VEGF-A isoforms (e.g. VEGF110, VEGF121, and VEGF165), thereby preventing binding of VEGF-A to its receptors VEGFR-1 and VEGFR-2. By inhibiting VEGF-A binding, brolucizumab suppresses endothelial cell proliferation, thereby reducing pathological neovascularisation and decreasing vascular permeability.
Pharmacodynamic effects
Wet AMD
In the HAWK and HARRIER studies, anatomical parameters related to leakage of blood and fluid that characterise choroidal neovascularisation (CNV) were part of the disease activity assessments guiding treatment decisions. Reductions in central subfield thickness (CST) and in presence of intraretinal/subretinal fluid (IRF/SRF) or sub-retinal pigment epithelium (sub-RPE) fluid were observed in patients treated with Beovu as early as 4 weeks after treatment initiation and up to week 48 and week 96.
At week 16, the reduction in CST was statistically significant on Beovu versus aflibercept in both studies (HAWK: -161 vs. -134 microns; HARRIER: -174 vs. -134 microns). This decrease from baseline in CST was also statistically significant at week 48 (HAWK: -173 vs. -144 microns; HARRIER: -194 vs. -144 microns), and maintained to the end of each study at week 96 (HAWK: -175 vs. -149 microns; HARRIER: -198 vs. -155 microns).
At week 16, the percentage difference in patients with IRF and/or SRF fluid was statistically significant on Beovu versus aflibercept in both studies (HAWK: 34% vs. 52%; HARRIER: 29% vs. 45%). This difference was also statistically significant at week 48 (HAWK: 31% vs. 45%; HARRIER: 26% vs. 44%), and maintained to the end of each study at week 96 (HAWK: 24% vs. 37%; HARRIER: 24% vs. 39%).
At week 16, the percentage difference in patients with sub-RPE fluid was statistically significant on Beovu versus aflibercept in both studies (HAWK: 19% vs. 27%; HARRIER: 16% vs. 24%). This difference was also statistically significant at week 48 (HAWK: 14% vs. 22%; HARRIER: 13% vs. 22%), and maintained to the end of each study at week 96 (HAWK: 11% vs. 15%; HARRIER: 17% vs. 22%).
In these studies, for patients treated with Beovu, reductions in CNV lesion size were observed as early as 12 weeks, and at weeks 48 and 96 after treatment initiation.
DME
In the KESTREL and KITE studies, related anatomical parameters were part of the disease activity assessments guiding treatment decisions. Reductions in CST and in presence of IRF/SRF were observed in patients treated with Beovu as early as 4 weeks after treatment initiation and up to week 52. These reductions were maintained up to week 100.
Clinical efficacy and safety
Wet AMD
The efficacy and safety of Beovu were assessed in two randomised, multicentre, double-masked, active-controlled Phase III studies (HAWK and HARRIER) in patients with neovascular (wet) AMD. A total of 1,817 patients were treated in these studies for two years (1 088 on Beovu and 729 on comparator aflibercept). Patient ages ranged from 50 to 97 years, with a mean age of 76 years.
In both studies, after the first three monthly doses (weeks 0, 4 and 8), brolucizumab patients were treated every 12 weeks, with the option of adjusting to a dosing interval every 8 weeks based on disease activity. Disease activity was assessed by a physician during the first 12-week interval (at weeks 16 and 20) and at each subsequent scheduled 12-weekly treatment visit. Patients who showed disease activity (e.g. decreased visual acuity, increased CST and/or presence of IRF/SRF or sub-RPE fluid) at any of these visits were adjusted to an 8-weekly treatment interval. The comparator aflibercept was administered every 8 weeks after the first 3 monthly doses.
Results
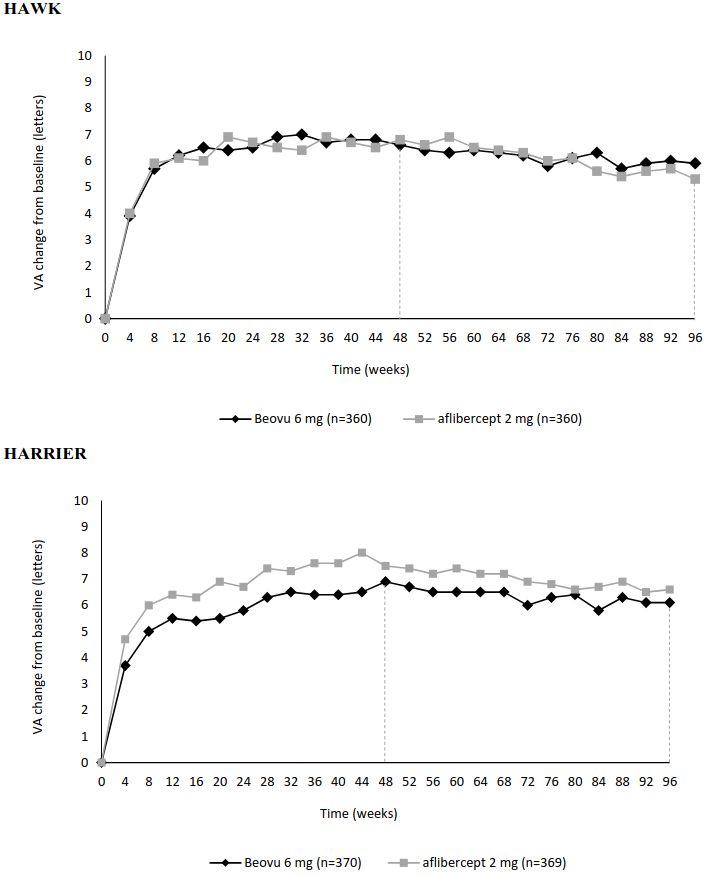
The primary efficacy endpoint for the studies was the change from baseline in best corrected visual acuity (BCVA) to week 48, as measured by the early treatment diabetic retinopathy study (ETDRS) letter score, with the primary objective being to demonstrate non-inferiority of Beovu versus aflibercept. In both studies, Beovu (administered in an every 12 weeks or an every 8 weeks regimen) demonstrated non-inferior efficacy to aflibercept 2 mg (administered every 8 weeks). The visual acuity gains observed in the first year were maintained in the second year. Detailed results of both studies are shown in Table 2 and in Figure 1 below.
Table 2. Visual acuity outcomes at weeks 48 and 96 in Phase III – HAWK and HARRIER studies:
| HAWK | HARRIER | ||||||
|---|---|---|---|---|---|---|---|
| Efficacy outcome | Week | Beovu (n=360) | Aflibercept 2 mg (n=360) | Difference (95% CI) brolucizumab – aflibercept | Beovu (n=370) | Aflibercept 2 mg (n=369) | Difference (95% CI) brolucizumab – aflibercept |
| Mean change from baseline in BCVA (measured by ETDRS letters score) | 48 | 6.6 (SE=0.71) | 6.8 (SE=0.71) | -0.2 (-2.1, 1.8) P<0.0001a | 6.9 (SE=0.61) | 7.6 (SE=0.61) | -0.7 (-2.4, 1.0) P<0.0001a |
| 36 – 48b | 6.7 (SE=0.68) | 6.7 (SE=0.68) | 0.0 (-1.9, 1.9) P<0.0001a | 6.5 (SE=0.58) | 7.7 (SE=0.58) | -1.2 (-2.8, 0.4) P=0.0003a | |
| 96 | 5.9 (SE=0.78) | 5.3 (SE=0.78) | 0.5 (-1.6, 2.7) | 6.1 (SE=0.73) | 6.6 (SE=0.73) | -0.4 (-2.5,1.6) | |
| % of patients who gained at least 15 letters of vision | 48 | 33.6 | 25.4 | 8.2 (2.2, 15.0) | 29.3 | 29.9 | -0.6 (-7.1, 5.8) |
| 96 | 34.2 | 27.0 | 7.2 (1.4, 13.8) | 29.1 | 31.5 | -2.4 (-8.8, 4.1) | |
| % of patients who lost visual acuity (%) (≥15 letters of BCVA loss) | 48 | 6.4 | 5.5 | 0.9 (-2.7, 4.3) | 3.8 | 4.8 | -1.0 (-3.9, 2.2) |
| 96 | 8.1 | 7.4 | 0.7 (-3.6, 4.6) | 7.1 | 7.5 | -0.4 (-3.8, 3.3) | |
BCVA: best corrected visual acuity; missing data are imputed using last observation carried forward (LOCF) method
ETDRS: early treatment diabetic retinopathy study
SE: standard error
a P-value referring to the non-inferiority hypothesis with a non-interiority margin of 4.0 letters.
b Key secondary endpoint, accounting for differences in timing of Beovu and aflibercept treatments.
Figure 1. Mean change in visual acuity from baseline to week 96 in HAWK and HARRIER studies:
These visual acuity gains were achieved with 56% and 51% of patients treated with Beovu on a 12-weekly dosing interval at week 48, and with 45% and 39% of patients at week 96 in HAWK and HARRIER, respectively. Among patients identified as eligible for the 12-weekly regimen during the first 12-week interval, 85% and 82% remained on the 12-weekly dosing interval up to week 48. Of patients on the 12-weekly interval at week 48, 82% and 75% remained on the 12-weekly dosing interval up to week 96.
Treatment effects in evaluable subgroups (e.g. age, gender, race, baseline visual acuity, baseline retinal thickness, lesion type, lesion size, fluid status) in each study were generally consistent with the results in the overall populations.
Disease activity was assessed by changes in visual acuity and/or anatomical parameters, including CST and/or presence of IRF/SRF or sub-RPE. Disease activity was assessed throughout the studies. Anatomical parameters of disease activity were decreased at week 48 and at week 96 for Beovu compared to aflibercept (see “Pharmacodynamic effects”).
The percentage difference in patients with disease activity at week 16 was statistically significant on Beovu versus aflibercept (24% vs 35% in HAWK, p=0.0013; 23% vs 32% in HARRIER, p=0.0021).
In both studies, Beovu demonstrated clinically meaningful increases from baseline in the pre-specified secondary efficacy endpoint of patient-reported outcomes, reported through the National Eye Institute Visual Function Questionnaire (NEI VFQ-25). The magnitude of these changes was similar to that seen in published studies, which corresponded to a 15-letter gain in BCVA. Patient-reported outcome benefits were maintained in the second year.
No clinically meaningful differences were found between Beovu and aflibercept in changes from baseline to week 48 in NEI VFQ-25 total score and subscales (general vision, ocular pain, near activities, distance activities, social functioning, mental health, role difficulties, dependency, driving, colour vision and peripheral vision).
The results of the Beovu arms of the HAWK and HARRIER studies, where Beovu was administered every 4 weeks (monthly) for the first 3 doses (loading) followed by maintenance dosing every 12 or 8 weeks, were replicated in a population pharmacokinetic/pharmacodynamic model simulation study where Beovu was administered every 6 weeks for the first 2 or 3 doses (loading) followed by maintenance dosing every 12 or 8 weeks.
DME
The efficacy and safety of Beovu were assessed in two randomised, multicentre, double-masked, activecontrolled Phase III studies (KESTREL and KITE) in patients with visual impairment due to diabetic macular oedema. A total of 926 patients were treated in these studies for two years (558 on brolucizumab and 368 on aflibercept 2 mg). Patient ages ranged from 23 to 87 years, with a mean age of 63 years.
In both studies, after the first five doses (weeks 0, 6, 12, 18 and 24), brolucizumab patients were treated every 12 weeks, with the option of adjusting to a dosing interval every 8 weeks based on disease activity. Disease activity was assessed by a physician during the first 12-week interval (at weeks 32 and 36) and at each subsequent scheduled treatment visit. Patients who showed disease activity (e.g. decreased visual acuity, increased CST) at any of these visits were adjusted to an every 8 weeks treatment interval. In year 2 of KITE, patients who showed no disease activity could be extended to a 16-week treatment interval. The comparator aflibercept was administered every 8 weeks after the first 5 monthly doses.
Results
The primary efficacy endpoint for the studies was the change from baseline in BCVA to week 52, as measured by the ETDRS letter score, with the primary objective being to demonstrate non-inferiority of Beovu versus aflibercept 2 mg. In both studies, Beovu (administered in an every 12 weeks or an every 8 weeks regimen) demonstrated non-inferior efficacy to aflibercept 2 mg (administered every 8 weeks).
The results of KESTREL and KITE also demonstrated non-inferiority of Beovu versus aflibercept 2 mg for the key secondary endpoint (average change from baseline in BVCA over the period week 40 to week 52).
The visual acuity gains observed in the first year were maintained in the second year.
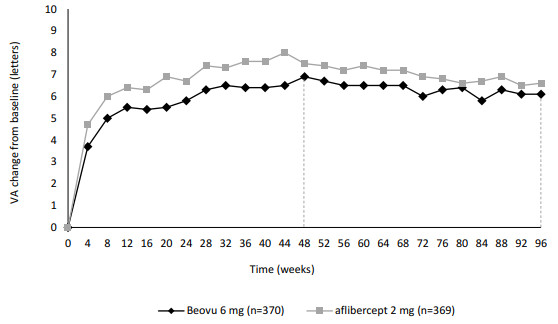
Detailed results of both studies are shown in Table 3 and in Figure 2 below.
Table 3. Visual acuity outcomes at weeks 52 and 100 in Phase III – KESTREL and KITE studies:
| KESTREL | KITE | ||||||
|---|---|---|---|---|---|---|---|
| Efficacy outcome | Week | Beovu (n=189) | Aflibercept 2 mg (n=187) | Difference (95% CI) brolucizumab – aflibercept | Beovu (n=179) | Aflibercept 2 mg (n=181) | Difference (95% CI) brolucizumab – aflibercept |
| Change from baseline in BCVA (measured by ETDRS letters score) – LS mean (SE) | 52 | 9.2 (0.57) | 10.5 (0.57) | -1.3 (-2.9, 0.3) P<0.001a | 10.6 (0.66) | 9.4 (0.66) | 1.2 (-0.6, 3.1) P<0.001a |
| 40-52 | 9.0 (0.53) | 10.5 (0.53) | -1.5 (-3.0, 0.0) P<0.001a | 10.3 (0.62) | 9.4 (0.62) | 0.9 (-0.9, 2.6) P<0.001a | |
| 100 | 8.8 (0.75) | 10.6 (0.75) | -1.7 (-3.8, 0.4) | 10.9 (0.85) | 8.4 (0.85) | 2.6 (0.2, 4.9) | |
| Gain of at least 15 letters in BCVA from baseline or BCVA ≥84 letters (%) | 52 | 36.0 | 40.1 | -4.1 (-13.3, 5.9) | 46.8 | 37.2 | 9.6 (-0.4, 20.2) |
| 100 | 39.2 | 42.2 | -3.0 (-12.5, 6.3) | 50.4 | 36.9 | 13.6 (3.3, 23.5) | |
BCVA: best corrected visual acuity; BCVA assessments after start of alternative DME treatment in the study eye were censored and replaced by the last value prior to start of this alternative treatment.
ETDRS: early treatment diabetic retinopathy study
LS: least-square
SE: standard error
a P-value referring to the non-inferiority hypothesis with a non-inferiority margin of 4.0 letters
Figure 2. Mean change in visual acuity from baseline to week 100 in KESTREL and KITE studies:
These visual acuity gains were achieved with 55% and 50% of patients treated with Beovu on a 12-weekly dosing interval at week 52, and 44% and 37% of patients treated with Beovu on a 12-weekly or 12-weekly/16-weekly dosing interval at week 100 in KESTREL and KITE, respectively. Among patients identified as eligible for the 12-weekly regimen during the first 12-week interval, approximately 70% remained on at least the 12-weekly interval at week 100 in both studies. In KITE, 25% of patients were treated with Beovu on a 16-weekly dosing interval at week 100.
Treatment effects in evaluable subgroups (e.g. age, gender, baseline HbA1c, baseline visual acuity, baseline central subfield thickness, DME lesion type, duration of DME since diagnosis, retinal fluid status) in each study were generally consistent with the results in the overall populations.
In KESTREL and KITE, disease activity was assessed throughout the studies by changes in visual acuity and/or anatomical parameters, including CST and/or presence of IRF/SRF. The reduction in CST from baseline was maintained up to week 100. At week 100, the proportion of patients with IRF/SRF was lower in patients treated with Beovu (42% KESTREL and 41% KITE) compared to patients treated with aflibercept 2 mg (54% KESTREL and 57% KITE).
Diabetic retinopathy severity score (DRSS) was assessed in the KESTREL and KITE studies. At baseline, 98.1% of patients in both KESTREL and KITE had gradable DRSS scores. Based on the pooled analysis, Beovu showed non-inferiority to aflibercept 2 mg in the proportion of subjects with at least a 2-step improvement from baseline in DRSS at week 52, using a non-inferiority margin of 10%. Estimated proportions were 28.9% and 24.9% in Beovu and aflibercept 2 mg, respectively, resulting in a treatment difference of 4.0% (95% CI: [-0.6, 8.6]). At week 100, the proportion of patients with a ≥2-step improvement from baseline to week 100 in the DRSS score was 32.8% with Beovu and 29.3% with aflibercept 2 mg in KESTREL and 35.8% with Beovu and 31.1% with aflibercept 2 mg in KITE.
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with Beovu in all subsets of the paediatric population in neovascular AMD and DME (see section 4.2 for information on paediatric use).
5.2. Pharmacokinetic properties
Beovu is administered directly into the vitreous to exert local effects in the eye.
Absorption and distribution
After intravitreal administration of 6 mg brolucizumab per eye to patients with nAMD, the geometirc mean Cmax of free brolucizumab in the plasma was 49.0 ng/ml (range: 8.97 to 548 ng/ml) and was attained in 1 day.
Biotransformation and elimination
Brolucizumab is a monoclonal antibody fragment and no metabolism studies have been conducted. As a single-chain antibody fragment, free brolucizumab is expected to undergo elimination through both target-mediated disposition via binding to free endogenous VEGF, passive renal elimination and metabolism via proteolysis.
After intravitreal injections, brolucizumab was eliminated with an apparent systemic half-life of 4.3 ± 1.9 days. Concentrations were generally near or below the quantitation limit (<0.5 ng/ml) approximately 4 weeks after dosing in most patients. Brolucizumab did not accumulate in the serum when administered intravitreally every 4 weeks.
Special populations
Elderly
There were no relevant differences in systemic pharmacokinetics following intravitreal injection in a study with 22 patients aged 65 to 74 years, 18 patients aged 75 to 84 years and 3 patients aged ≥85 years.
Renal impairment
The systemic pharmacokinetics of brolucizumab was evaluated in nAMD patients with normal renal function (≥90 ml/min [n=21]), with mild (60 to <90 ml/min [n=22]) or moderate (30 to <60 ml/min [n=7]) renal impairment. While the mean systemic clearance values for patients with mild or moderate renal impairment were generally lower than patients with normal renal function, no significant impact of mild and moderate renal impairment on the overall systemic exposure to brolucizumab was observed. No patients with severe (<30 ml/min) renal impairment were studied.
Hepatic impairment
Brolucizumab has not been studied in patients with hepatic impairment. Mild to severe hepatic impairment should have no impact on the overall systemic exposure to brolucizumab, because metabolism occurs via proteolysis and does not depend on hepatic function.
5.3. Preclinical safety data
No studies have been conducted on the carcinogenic or mutagenic potential of brolucizumab.
In pregnant cynomolgus monkeys, brolucizumab was administered once every 4 weeks by intravitreal injection at dose levels resulting in maximal systemic exposures 6-fold higher than those in humans at the maximum recommended dose (based on serum Cmax). There was no impact on embryofoetal development, pregnancy or parturition, or on the survival, growth or postnatal development of offspring. Nevertheless, based on its pharmacological effect, brolucizumab should be regarded as potentially teratogenic and embryo-foetotoxic.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.