BEROTEC Inhaler Ref.[50446] Active ingredients:
Source: Health Products Regulatory Authority (ZA) Revision Year: 2022 Publisher: Ingelheim Pharmaceuticals (Pty) Ltd, 407 Pine Avenue, Randburg, South Africa
4.1. Therapeutic indications
a) Symptomatic treatment of acute asthma attacks.
b) Prophylaxis of exercise induced asthma.
c) Symptomatic treatment of bronchial asthma and other conditions with reversible airway narrowing e.g. chronic obstructive bronchitis.
Concomitant anti-inflammatory therapy should be considered for patients with bronchial asthma and steroid responsive chronic obstructive pulmonary disease (COPD).
4.2. Posology and method of administration
Acute asthma episodes
One puff is sufficient for prompt symptom relief in many cases. In more severe cases, if breathing has not noticeably improved after 5 minutes, a second dose may be taken.
If an attack has not been relieved by 2 puffs, further puffs may be required. In these cases, patients should consult the doctor or the nearest hospital immediately.
Prophylaxis of exercise induced asthma
One to two puffs for each administration, up to a maximum of eight puffs per day.
Bronchial asthma and other conditions with reversible airways narrowing
If repeated dosing is required, one to two puffs for each administration, up to a maximum of eight puffs per day.
In children BEROTEC 100 HFA should be used only on medical advice and under the supervision of an adult.
DO NOT EXCEED THE RECOMMENDED DOSE.
4.9. Overdose
Symptoms
The expected symptoms with overdosage are those of excessive beta-adrenergic-stimulation, including exaggeration of the known pharmacologic effects, i.e. any of the symptoms listed under side-effects, the most prominent being tachycardia, palpitation, tremor, hypertension, hypotension, widening of the pulse pressure, anginal pain, arrhythmias and flushing.
Treatment
Administration of sedatives, tranquillizers, in severe cases intensive therapy. Beta-receptor blockers, preferably beta1-receptor blockers, are suitable as specific antidotes; however, a possible increase in bronchial obstruction must be taken into account and the dose should be adjusted carefully in patients suffering from bronchial asthma.
Further treatment should be symptomatic and supportive.
6.4. Special precautions for storage
Store at or below 30°C in a dry place out of the reach of children.
Do not force container open.
6.5. Nature and contents of container
Metered aerosol complete with a mouthpiece.
Canister of 10 ml, providing 200 metered doses.
6.6. Special precautions for disposal and other handling
Instructions for use/handling
The correct administration of the metered aerosol is essential for successful therapy.
Depress the valve twice before the apparatus is used for the first time.
Before each use the following directions should be followed:
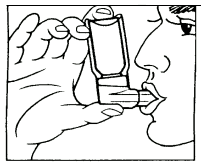
1. Remove the protective plastic cap.
(Fig 1.)
2. Breathe out deeply.
3. Hold the metered aerosol as shown in Fig. 1, and close the lips over the mouthpiece. The arrow and the base of the container should be pointing upwards.
4. Breathe in as deeply as possible, pressing the base of the container firmly at the same time, this releases one metered dose. Hold your breath for a few seconds, then remove the mouthpiece and breathe out. The same action should be repeated for a second inhalation.
5. Replace the protective cap after use.
6. After not using the metered aerosol for three days the valve has to be actuated once.
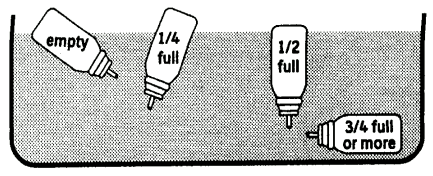
The container is not transparent. It is not therefore possible to see when it is empty. The aerosol will deliver 200 doses. When these have all been used the aerosol may still appear to contain a small amount of fluid. The aerosol should, however, be replaced because you may not get the right amount of treatment.
The amount of treatment in your aerosol can be checked as follows: Remove the aerosol from the plastic mouthpiece and put the aerosol into a container of water.
The contents of the aerosol can be estimated by observing its position in the water (see Fig. 2).
(Fig. 2)
The mouthpiece can be washed with warm water and should always be kept clean. If soap or detergent is used, the mouthpiece should be thoroughly rinsed in clean water.
WARNING: The plastic mouthpiece has been specially designed for use with BEROTEC 100 HFA to ensure that you always get the right amount of the medicine. The mouthpiece must never be used with any other metered aerosol nor must the BEROTEC 100 HFA aerosol be used with any mouthpiece other than the one supplied with the product.
The container is under pressure and should on no account be opened by force or exposed to temperatures exceeding 50°C.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.