BEXSERO Suspension for injection Ref.[50804] Active ingredients: Neisseria meningitidis OMPC
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: GSK Vaccines S.r.l., Via Fiorentina 1, 53100 Siena, Italy
4.1. Therapeutic indications
Bexsero is indicated for active immunisation of individuals from 2 months of age and older against invasive meningococcal disease caused by Neisseria meningitidis group B. The impact of invasive disease in different age groups as well as the variability of antigen epidemiology for group B strains in different geographical areas should be considered when vaccinating. See section 5.1 for information on protection against specific group B strains. The use of this vaccine should be in accordance with official recommendations.
4.2. Posology and method of administration
Posology
Table 1. Summary of posology:
| Age at first dose | Primary Immunisation | Intervals between Primary Doses | Booster |
|---|---|---|---|
| Infants, 2 months to 5 monthsa | Three doses each of 0.5 ml | Not less than 1 month | Yes, one dose between 12 and 15 months of age with an interval of at least 6 months between the primary series and booster doseb,c |
| Two doses each of 0.5 ml | Not less than 2 months | ||
| Infants, 6 months to 11 months | Two doses each of 0.5 ml | Not less than 2 months | Yes, one dose in the second year of life with an interval of at least 2 months between the primary series and booster dosec |
| Children, 12 months to 23 months | Two doses each of 0.5 ml | Not less than 2 months | Yes, one dose with an interval of 12 months to 23 months between the primary series and booster dosec |
| Children, 2 years to 10 years | Two doses each of 0.5 ml | Not less than 1 month | A booster dose should be considered in individuals at continued risk of exposure to meningococcal disease, based on official recommendationsd |
| Adolescents (from 11 years) and adults* |
a The first dose should be given no earlier than 2 months of age. The safety and efficacy of Bexsero in infants less than 8 weeks of age has not yet been established. No data are available.
b In case of delay, the booster should not be given later than 24 months of age.
c See section 5.1. The need for and timing of further booster doses has not yet been determined.
d See section 5.1.
* There are no data in adults above 50 years of age.
Method of administration
The vaccine is given by deep intramuscular injection, preferably in the anterolateral aspect of the thigh in infants or in the deltoid muscle region of the upper arm in older subjects.
Separate injection sites must be used if more than one vaccine is administered at the same time.
The vaccine must not be injected intravenously, subcutaneously or intradermally and must not be mixed with other vaccines in the same syringe.
For instructions on the handling of the vaccine before administration, see section 6.6.
4.9. Overdose
Experience of overdose is limited. In the event of overdose, monitoring of vital functions and possible symptomatic treatment is recommended.
6.3. Shelf life
4 years.
6.4. Special precautions for storage
Store in a refrigerator (2°C-8°C).
Do not freeze.
Store in the original package in order to protect from light.
6.5. Nature and contents of container
0.5 ml of suspension in a pre-filled syringe (type I glass) with a plunger stopper (butyl rubber) and with a rubber tip cap.
The tip cap and rubber plunger stopper of the pre-filled syringe are made with synthetic rubber.
Pack sizes of 1 and 10, with or without needles.
Not all pack sizes may be marketed.
6.6. Special precautions for disposal and other handling
Upon storage a fine off-white deposit may be observed in the pre-filled syringe containing the suspension.
Before use, the pre-filled syringe should be well shaken in order to form a homogeneous suspension.
The vaccine should be visually inspected for particulate matter and discoloration prior to administration. In the event of any foreign particulate matter and/or variation of physical aspect being observed, do not administer the vaccine. If two needles of different lengths are provided in the pack, choose the appropriate needle to ensure an intramuscular administration.
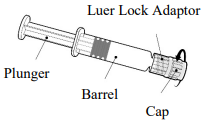
Instructions for the pre-filled syringe
Hold the syringe by the barrel, not by the plunger.
Unscrew the syringe cap by twisting it anticlockwise.
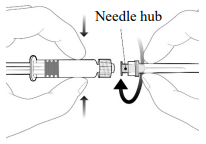
To attach the needle, connect the hub to the Luer Lock Adaptor and rotate a quarter turn clockwise until you feel it lock.
Do not pull the syringe plunger out of the barrel. If it happens, do not administer the vaccine.
Disposal
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.