BIKTARVY Film-coated tablet Ref.[6515] Active ingredients: Bictegravir Emtricitabine Emtricitabine, Tenofovir alafenamide and Bictegravir Tenofovir alafenamide
Source: European Medicines Agency (EU) Revision Year: 2022 Publisher: Gilead Sciences Ireland UC, Carrigtohill, County Cork, T45 DP77, Ireland
4.3. Contraindications
Hypersensitivity to the active substances or to any of the excipients listed in section 6.1.
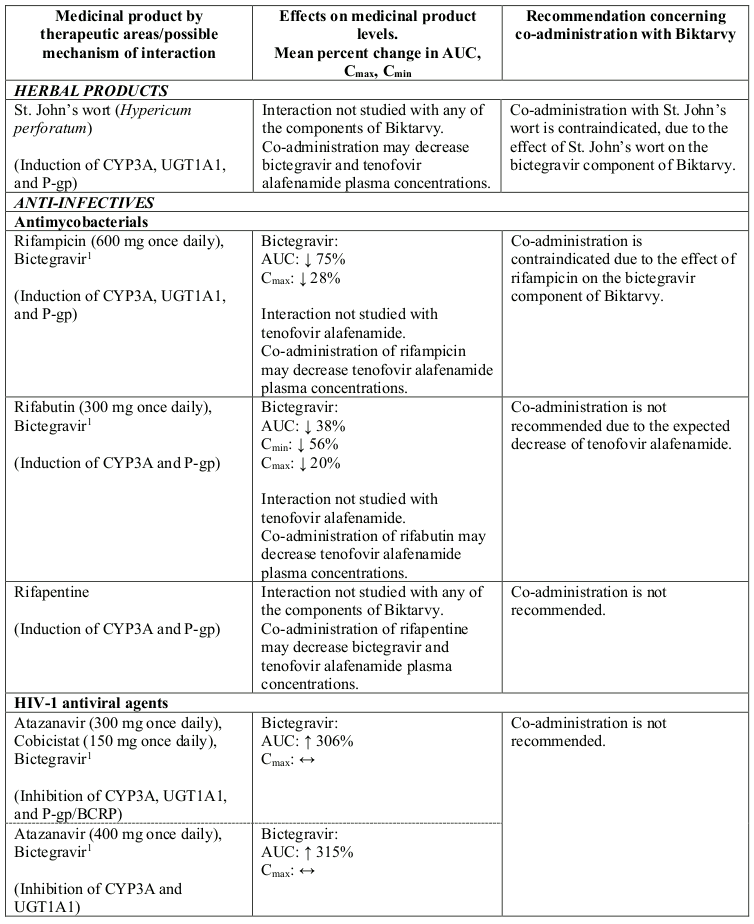
Co-administration with rifampicin and St John’s Wort (Hypericum perforatum) (see section 4.5).
4.4. Special warnings and precautions for use
While effective viral suppression with antiretroviral therapy has been proven to substantially reduce the risk of sexual transmission, a residual risk cannot be excluded. Precautions to prevent transmission should be taken in accordance with national guidelines.
Patients co-infected with HIV and hepatitis B or C virus
Patients with chronic hepatitis B or C treated with antiretroviral therapy are at an increased risk for severe and potentially fatal hepatic adverse reactions.
There are limited safety and efficacy data for Biktarvy in patients co-infected with HIV-1 and hepatitis C virus (HCV).
Biktarvy contains tenofovir alafenamide, which is active against hepatitis B virus (HBV).
Discontinuation of Biktarvy therapy in patients co-infected with HIV and HBV may be associated with severe acute exacerbations of hepatitis. Patients co-infected with HIV and HBV who discontinue Biktarvy should be closely monitored with both clinical and laboratory follow-up for at least several months after stopping treatment.
Liver disease
The safety and efficacy of Biktarvy in patients with significant underlying liver disorders have not been established.
Patients with pre-existing liver dysfunction, including chronic active hepatitis, have an increased frequency of liver function abnormalities during combination antiretroviral therapy (CART) and should be monitored according to standard practice. If there is evidence of worsening liver disease in such patients, interruption or discontinuation of treatment must be considered.
Weight and metabolic parameters
An increase in weight and in levels of blood lipids and glucose may occur during antiretroviral therapy. Such changes may in part be linked to disease control and life style. For lipids, there is in some cases evidence for a treatment effect, while for weight gain there is no strong evidence relating this to any particular treatment. For monitoring of blood lipids and glucose reference is made to established HIV treatment guidelines. Lipid disorders should be managed as clinically appropriate.
Mitochondrial dysfunction following exposure in utero
Nucleos(t)ide analogues may impact mitochondrial function to a variable degree, which is most pronounced with stavudine, didanosine and zidovudine. There have been reports of mitochondrial dysfunction in HIV negative infants exposed in utero and/or postnatally to nucleoside analogues; these have predominantly concerned treatment with regimens containing zidovudine. The main adverse reactions reported are haematological disorders (anaemia, neutropenia) and metabolic disorders (hyperlactatemia, hyperlipasemia). These events have often been transitory. Late onset neurological disorders have been reported rarely (hypertonia, convulsion, abnormal behaviour). Whether such neurological disorders are transient or permanent is currently unknown. These findings should be considered for any child exposed in utero to nucleos(t)ide analogues, who present with severe clinical findings of unknown etiology, particularly neurologic findings. These findings do not affect current national recommendations to use antiretroviral therapy in pregnant women to prevent vertical transmission of HIV.
Immune Reactivation Syndrome
In HIV-infected patients with severe immune deficiency at the time of institution of CART, an inflammatory reaction to asymptomatic or residual opportunistic pathogens may arise and cause serious clinical conditions, or aggravation of symptoms. Typically, such reactions have been observed within the first few weeks or months of initiation of CART. Relevant examples include cytomegalovirus retinitis, generalised and/or focal mycobacterial infections, and Pneumocystis jirovecii pneumonia. Any inflammatory symptoms should be evaluated and treatment instituted when necessary.
Autoimmune disorders (such as Graves' disease and autoimmune hepatitis) have also been reported to occur in the setting of immune reactivation; however, the reported time to onset is more variable and these events can occur many months after initiation of treatment.
Opportunistic infections
Patients should be advised that Biktarvy or any other antiretroviral therapy does not cure HIV infection and that they may still develop opportunistic infections and other complications of HIV infection. Therefore patients should remain under close clinical observation by physicians experienced in the treatment of patients with HIV associated diseases.
Osteonecrosis
Although the aetiology is considered to be multifactorial (including corticosteroid use, alcohol consumption, severe immunosuppression, higher body mass index), cases of osteonecrosis have been reported particularly in patients with advanced HIV disease and/or long-term exposure to CART. Patients should be advised to seek medical advice if they experience joint aches and pain, joint stiffness or difficulty in movement.
Nephrotoxicity
A potential risk of nephrotoxicity resulting from chronic exposure to low levels of tenofovir due to dosing with tenofovir alafenamide cannot be excluded (see section 5.3).
It is recommended that renal function is assessed in all patients prior to, or when initiating, therapy with Biktarvy and that it is also monitored during therapy in all patients as clinically appropriate. In patients who develop clinically significant decreases in renal function, or evidence of proximal renal tubulopathy, discontinuation of Biktarvy should be considered.
Patients with end stage renal disease on chronic haemodialysis
Biktarvy should generally be avoided but may be used in adults with end stage renal disease (estimated CrCl <15 mL/min) on chronic haemodialysis if the potential benefits outweigh the potential risks (see section 4.2). In a study of emtricitabine + tenofovir alafenamide in combination with elvitegravir + cobicistat as a fixed-dose combination tablet (E/C/F/TAF) in HIV-1 infected adults with end stage renal disease (estimated CrCl <15 mL/min) on chronic haemodialysis, efficacy was maintained through 96 weeks but emtricitabine exposure was significantly higher than in patients with normal renal function. Efficacy was also maintained in the extension phase of the study in which 10 patients switched to Biktarvy for 48 weeks. Although no additional adverse reactions were identified, the implications of increased emtricitabine exposure remain uncertain (see sections 4.8 and 5.2).
Co-administration of other medicinal products
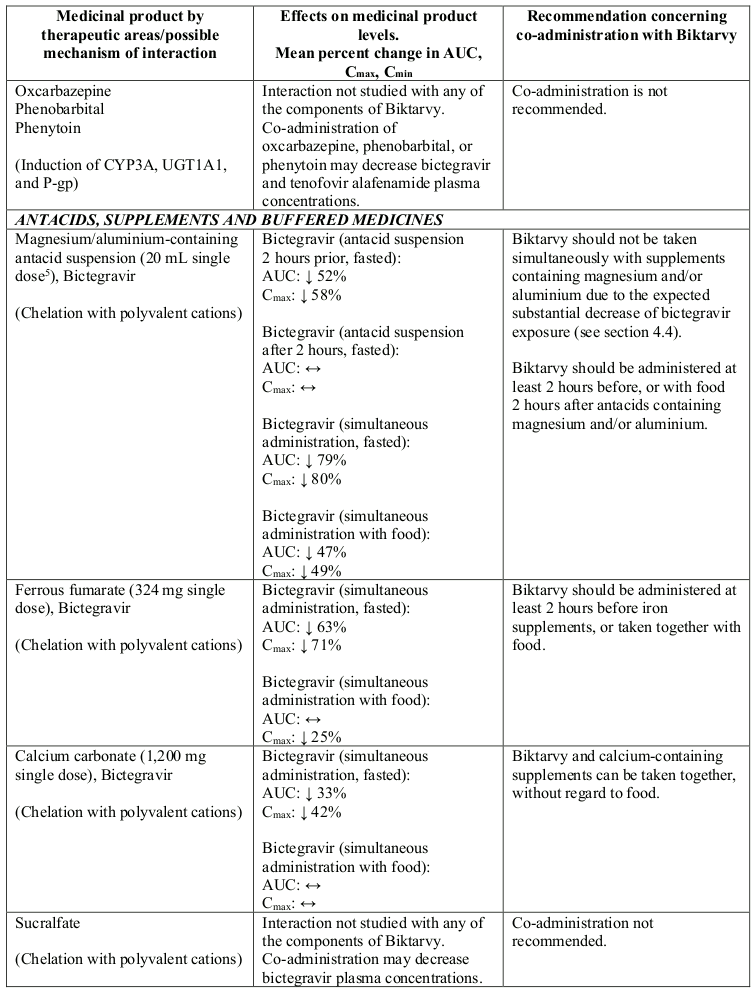
Biktarvy should not be co-administered simultaneously with magnesium/aluminium-containing antacids or iron supplements under fasted conditions. Biktarvy should be administered at least 2 hours before, or with food 2 hours after antacids containing magnesium and/or aluminium. Biktarvy should be administered at least 2 hours before iron supplements, or taken together with food (see section 4.5).
Some medicinal products are not recommended for co-administration with Biktarvy: atazanavir, carbamazepine, ciclosporin (IV or oral use), oxcarbazepine, phenobarbital, phenytoin, rifabutin, rifapentine, or sucralfate.
Biktarvy should not be co-administered with other antiretroviral medicinal products.
Excipients
This medicine contains less than 1 mmol sodium (23 mg) per tablet, that is to say essentially ‘sodium-free’.
4.5. Interaction with other medicinal products and other forms of interaction
Interaction studies have only been performed in adults.
Biktarvy should not be administered concomitantly with medicinal products containing tenofovir alafenamide, tenofovir disoproxil, lamivudine or adefovir dipivoxil used for the treatment of HBV infection.
Bictegravir
Bictegravir is a substrate of CYP3A and UGT1A1. Co-administration of bictegravir and medicinal products that potently induce both CYP3A and UGT1A1, such as rifampicin or St. John’s wort, may significantly decrease plasma concentrations of bictegravir, which may result in a loss of therapeutic effect of Biktarvy and development of resistance, therefore co-administration is contraindicated (see section 4.3). Co-administration of bictegravir with medicinal products that potently inhibit both CYP3A and UGT1A1, such as atazanavir, may significantly increase plasma concentrations of bictegravir, therefore co-administration is not recommended.
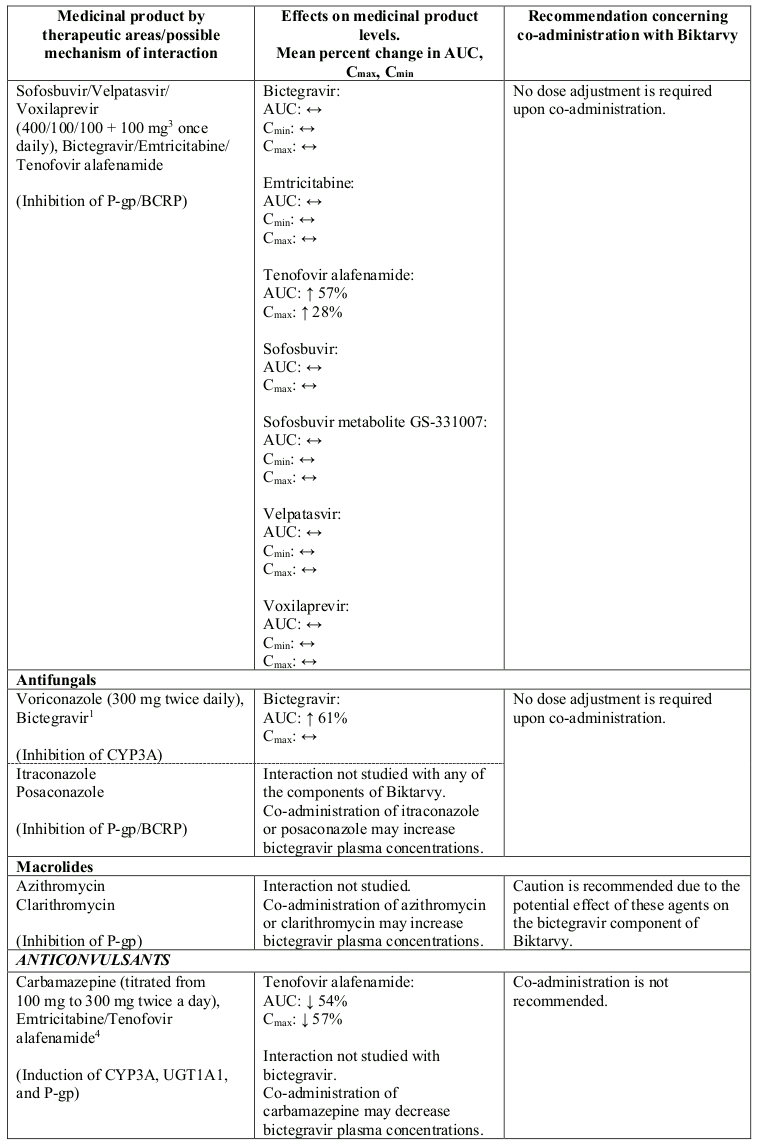
Bictegravir is both a P-gp and a BCRP substrate. The clinical relevance of this feature is not established. Therefore, caution is recommended when bictegravir is combined with medicinal products known to inhibit P-gp and/or BCRP (e.g. macrolides, ciclosporin, verapamil, dronedarone, glecaprevir/pibrentasvir) (see also table below).
Bictegravir inhibits organic cation transporter 2 (OCT2) and multidrug and toxin extrusion transporter 1 (MATE1) in vitro. Co-administration of Biktarvy with the OCT2 and MATE1 substrate metformin did not result in a clinically significant increase in metformin exposure. Biktarvy may be co-administered with substrates of OCT2 and MATE1.
Bictegravir is not an inhibitor or inducer of CYP in vivo.
Emtricitabine
In vitro and clinical pharmacokinetic drug-drug interaction studies have shown that the potential for CYP-mediated interactions involving emtricitabine with other medicinal products is low. Co-administration of emtricitabine with medicinal products that are eliminated by active tubular secretion may increase concentrations of emtricitabine, and/or the co-administered medicinal product. Medicinal products that decrease renal function may increase concentrations of emtricitabine.
Tenofovir alafenamide
Tenofovir alafenamide is transported by P-glycoprotein (P–gp) and breast cancer resistance protein (BCRP). Co-administration of Biktarvy with medicinal products that strongly affect P-gp and BCRP activity may lead to changes in tenofovir alafenamide absorption. Medicinal products that induce P-gp activity (e.g. rifabutin, carbamazepine, phenobarbital) are expected to decrease the absorption of tenofovir alafenamide, resulting in decreased plasma concentration of tenofovir alafenamide, which may lead to loss of therapeutic effect of Biktarvy and development of resistance. Co-administration of Biktarvy with other medicinal products that inhibit P-gp and BCRP may increase the absorption and plasma concentration of tenofovir alafenamide.
Tenofovir alafenamide is not an inhibitor or inducer of CYP3A in vivo.
Other interactions
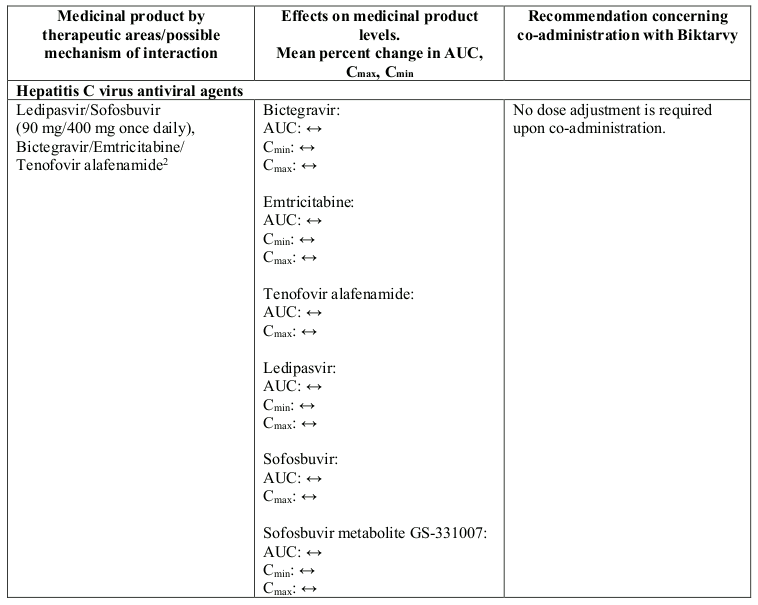
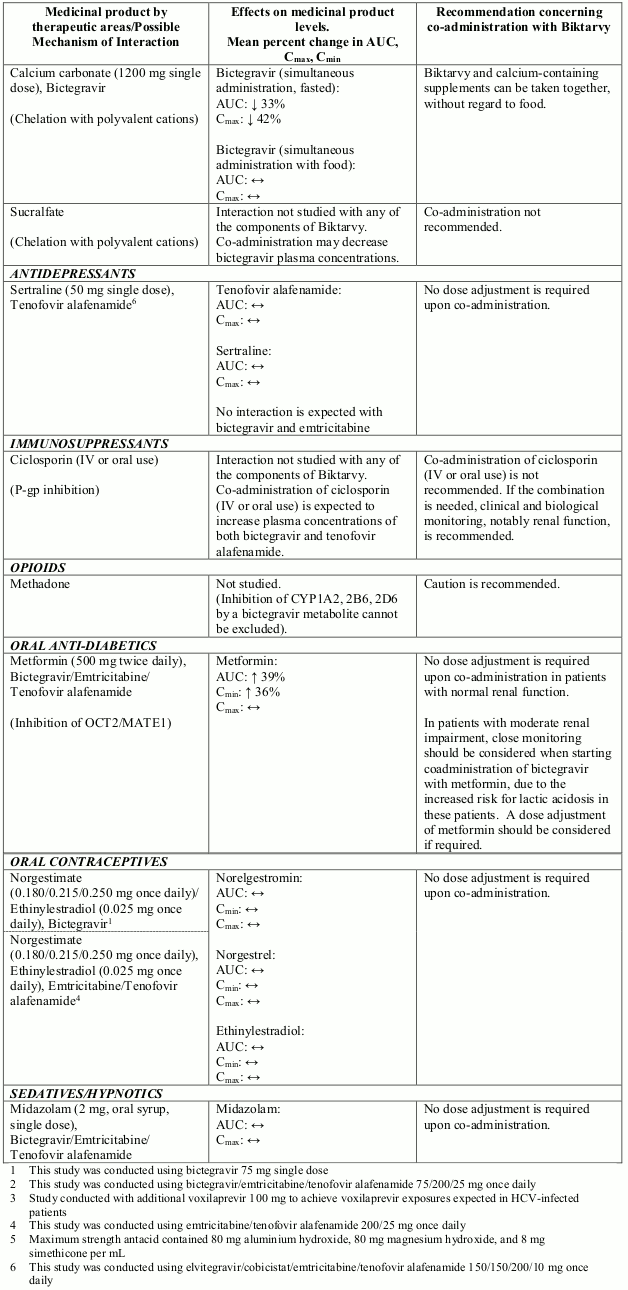
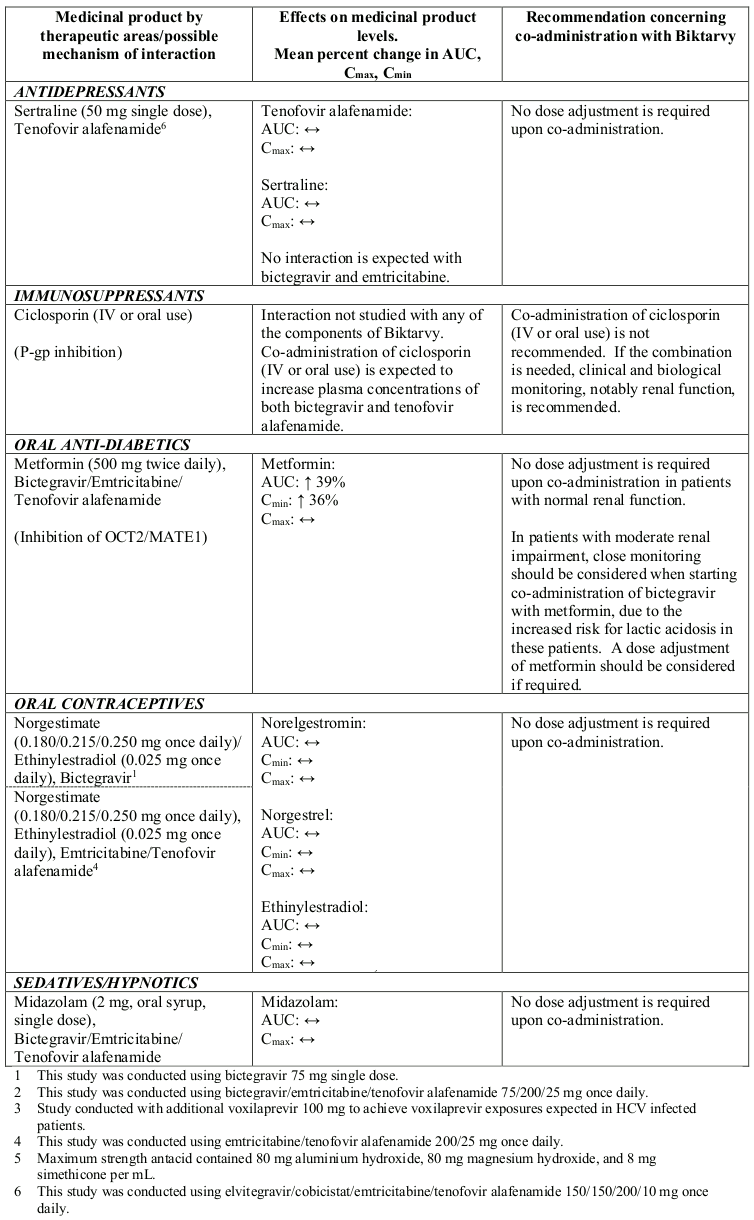
Interactions between Biktarvy or its individual component(s) and co-administered medicinal products are listed in Table 1 below (increase is indicated as “↑”, decrease as “↓” and no change as “↔”; all No Effect Boundaries are 70%-143%).
Table 1. Interactions between Biktarvy or its individual component(s) and other medicinal products:
Based on drug interaction studies conducted with Biktarvy or the components of Biktarvy, no clinically significant drug interactions are expected with: amlodipine, atorvastatin, buprenorphine, drospirenone, famciclovir, famotidine, fluticasone, naloxone, norbuprenorphin, omeprazole or rosuvastatin.
4.6. Fertility, pregnancy and lactation
Pregnancy
There are no or limited data (less than 300 pregnancy outcomes) from the use of bictegravir or tenofovir alafenamide in pregnant women. A large amount of data on pregnant women (more than 1,000 exposed outcomes) indicate no malformative nor foetal/neonatal toxicity associated with emtricitabine.
Animal studies do not indicate direct or indirect harmful effects of emtricitabine with respect to fertility parameters, pregnancy, foetal development, parturition or postnatal development. Studies of bictegravir and tenofovir alafenamide, administered separately, in animals have shown no evidence of harmful effects on fertility parameters, pregnancy, or foetal development (see section 5.3).
Biktarvy should be used during pregnancy only if the potential benefit justifies the potential risk to the foetus.
Breast-feeding
It is not known whether bictegravir or tenofovir alafenamide is excreted in human milk. Emtricitabine is excreted in human milk. In animal studies, bictegravir was detected in the plasma of nursing rat pups likely due to the presence of bictegravir in milk, without effects on nursing pups. In animal studies it has been shown that tenofovir is excreted in milk.
There is insufficient information on the effects of all the components of Biktarvy in newborns/infants, therefore Biktarvy should not be used during breast-feeding.
In order to avoid transmission of HIV to the infant it is recommended that HIV-infected women do not breast-feed their infants under any circumstances.
Fertility
No human data on the effect of Biktarvy on fertility are available. Animal studies indicate no effects of bictegravir, emtricitabine or tenofovir alafenamide on mating or fertility (see section 5.3).
4.7. Effects on ability to drive and use machines
Patients should be informed that dizziness has been reported during treatment with the components of Biktarvy (see section 4.8).
4.8. Undesirable effects
Summary of the safety profile
The assessment of adverse reactions is based on safety data from across all Phase 2 and 3 studies with Biktarvy and from post-marketing experience. In clinical studies of treatment-naïve patients receiving Biktarvy, the most frequently reported adverse reactions in the double-blind phase (Week 144) were headache (5%), diarrhoea (5%) and nausea (4%).
Tabulated summary of adverse reactions
The adverse reactions in Table 2 are listed by system organ class and frequency. Frequencies are defined as follows: very common (≥1/10), common (≥1/100 to <1/10) and uncommon (≥1/1,000 to <1/100).
Table 2. Tabulated list of adverse reactions1:
| Frequency | Adverse reaction |
|---|---|
| Blood and lymphatic system disorders | |
| Uncommon | anaemia2 |
| Psychiatric disorders | |
| Common | depression, abnormal dreams |
| Uncommon | suicidal ideation, suicide attempt (particularly in patients with a pre-existing history of depression or psychiatric illness), anxiety, sleep disorders |
| Nervous system disorders | |
| Common | headache, dizziness |
| Gastrointestinal disorders | |
| Common | diarrhoea, nausea |
| Uncommon | vomiting, abdominal pain, dyspepsia, flatulence |
| Hepatobiliary disorders | |
| Uncommon | hyperbilirubinaemia |
| Skin and subcutaneous tissue disorders | |
| Uncommon | angioedema3,4, rash, pruritus, urticaria4 |
| Rare | Stevens-Johnson syndrome5 |
| Musculoskeletal and connective tissue disorders | |
| Uncommon | arthralgia |
| General disorders and administration site conditions | |
| Common | fatigue |
1 With the exception of angioedema, anaemia, urticaria and Stevens-Johnson syndrome (see footnotes 2-5), all adverse reactions were identified from Biktarvy clinical studies. The frequencies were derived from the double-blind phase (Week 144) of Phase 3 Biktarvy clinical studies in treatment-naïve patients (GS-US-380-1489 and GS-US-380-1490).
2 This adverse reaction was not observed in the clinical studies of emtricitabine + tenofovir alafenamide-containing products but identified from clinical studies or post-marketing experience for emtricitabine when used with other antiretrovirals.
3 This adverse reaction was identified through post-marketing surveillance for emtricitabine-containing products.
4 This adverse reaction was identified through post-marketing surveillance for tenofovir alafenamide-containing products.
5 This adverse reaction was identified through post-marketing surveillance for Biktarvy. The frequency has been calculated using 3/X, where X represent the cumulative number of subjects exposed to Biktarvy in clinical trials (N=3963).
Description of selected adverse reactions
Metabolic parameters
Weight and levels of blood lipids and glucose may increase during antiretroviral therapy (see section 4.4).
Immune Reactivation Syndrome
In HIV infected patients with severe immune deficiency at the time of initiation of CART, an inflammatory reaction to asymptomatic or residual opportunistic infections may arise. Autoimmune disorders (such as Graves' disease and autoimmune hepatitis) have also been reported; however, the reported time to onset is more variable and these events can occur many months after initiation of treatment (see section 4.4).
Osteonecrosis
Cases of osteonecrosis have been reported, particularly in patients with generally acknowledged risk factors, advanced HIV disease or long-term exposure to CART. The frequency of this is unknown (see section 4.4).
Changes in serum creatinine
Bictegravir has been shown to increase serum creatinine due to inhibition of tubular secretion of creatinine, however these changes are not considered to be clinically relevant since they do not reflect a change in glomerular filtration rate. Increases in serum creatinine occurred by Week 4 of treatment and remained stable through Week 144. In Studies GS-US-380-1489 and GS-US-380-1490, median (Q1, Q3) serum creatinine increased by 0.11 (0.03, 0.19) mg/dL (9.7 [2.7, 16.8] µmol/L), 0.11 (0.04, 0.19) mg/dL (9.7 [3.5, 16.8] µmol/L), and 0.12 (0.06, 0.21) mg/dL (10.6 [5.3, 18.6] μmol/L) from baseline to Week 144 in the Biktarvy, abacavir/dolutegravir/lamivudine, and dolutegravir + emtricitabine/tenofovir alafenamide groups, respectively. There were no discontinuations due to renal adverse events through Week 144 in patients administered Biktarvy in clinical studies.
Changes in bilirubin
In Studies GS-US-380-1489 and GS-US-380-1490, total bilirubin increases were observed in 17% of treatment-naïve patients administered Biktarvy through Week 144. Increases were primarily Grade 1 (12%) and Grade 2 (4%) (≥1.0 to 2.5 x Upper Limit of Normal [ULN]), and were not associated with hepatic adverse reactions or other liver related laboratory abnormalities. Five patients administered Biktarvy (1%) had grade 3 bilirubin increases that were not considered related to study drug. There were no discontinuations due to hepatic adverse events through Week 144 in Biktarvy clinical studies.
Other special populations
Patients co-infected with hepatitis B
In 16 HIV/HBV co-infected adults administered Biktarvy (8 HIV/HBV treatment-naïve adults in Study GS-US-380-1490; 8 HIV/HBV suppressed adults in Study GS-US-380-1878), the safety profile of Biktarvy was similar to that in patients with HIV-1 monoinfection (see section 5.1).
Elderly
Studies GS-US-380-1844, GS-US-380-1878 and the dedicated Study GS-US-380-4449 in patients ≥65 years old (evaluation of 86 HIV-1 infected, virologically-suppressed subjects ≥65 years old) included 111 patients aged ≥65 years who received Biktarvy. In these patients, no differences in the safety profile of Biktarvy were observed.
Patients with renal impairment
The safety of emtricitabine + tenofovir alafenamide was evaluated in a single arm, open-label clinical study (GS-US-292-1825), in which 55 virologically-suppressed HIV-1 infected patients with end stage renal disease (eGFRCG <15 mL/min) on chronic haemodialysis received emtricitabine + tenofovir alafenamide in combination with elvitegravir + cobicistat as a fixed-dose combination tablet for 96 weeks. In an extension phase of Study GS-US-292-1825, 10 patients switched to Biktarvy for 48 weeks. No additional adverse reactions were identified in patients with end stage renal disease on chronic haemodialysis in this study (see sections 4.4 and 5.2).
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system listed in Appendix V.
6.2. Incompatibilities
Not applicable.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.