BLINCYTO Powder and solution for solution for infusion Ref.[6566] Active ingredients: Blinatumomab
Source: European Medicines Agency (EU) Revision Year: 2025 Publisher: Amgen Europe B.V., Minervum 7061, 4817 ZK Breda, The Netherlands
4.1. Therapeutic indications
BLINCYTO is indicated as monotherapy for the treatment of adults with CD19 positive relapsed or refractory B-cell precursor acute lymphoblastic leukaemia (ALL). Patients with Philadelphia chromosome-positive B-cell precursor ALL should have failed treatment with at least 2 tyrosine kinase inhibitors (TKIs) and have no alternative treatment options.
BLINCYTO is indicated as monotherapy for the treatment of adults with Philadelphia chromosome-negative CD19 positive B-cell precursor ALL in first or second complete remission with minimal residual disease (MRD) greater than or equal to 0.1%.
BLINCYTO is indicated as monotherapy for the treatment of paediatric patients aged 1 month or older with Philadelphia chromosome-negative CD19 positive B-cell precursor ALL which is refractory or in relapse after receiving at least two prior therapies or in relapse after receiving prior allogeneic haematopoietic stem cell transplantation.
BLINCYTO is indicated as monotherapy for the treatment of paediatric patients aged 1 month or older with high-risk first relapsed Philadelphia chromosome-negative CD19 positive B-cell precursor ALL as part of the consolidation therapy (see section 4.2).
BLINCYTO is indicated as monotherapy as part of consolidation therapy for the treatment of adult patients with newly diagnosed Philadelphia chromosome negative CD19 positive B-cell precursor ALL.
4.2. Posology and method of administration
Treatment should be initiated under the direction of and supervised by physicians experienced in the treatment of haematological malignancies. Patients treated with BLINCYTO should be given the Educational Brochure for Patients and Caregivers and the Patient Card.
For the treatment of relapsed or refractory B-cell precursor ALL, hospitalisation is recommended for initiation at a minimum for the first 9 days of the first cycle and the first 2 days of the second cycle.
For the treatment of Philadelphia chromosome-negative MRD positive B-cell precursor ALL, hospitalisation is recommended at a minimum for the first 3 days of the first cycle and the first 2 days of subsequent cycles.
For the treatment of B-cell precursor ALL in the consolidation phase, hospitalisation is recommended for the first 3 days of the first cycle and the first 2 days of the second cycle.
In patients with a history or presence of clinically relevant central nervous system (CNS) pathology (see section 4.4), hospitalisation is recommended at a minimum for the first 14 days of the first cycle.
In the second cycle, hospitalisation is recommended at a minimum for 2 days, and clinical judgment should be based on tolerance to BLINCYTO in the first cycle. Caution should be exercised as cases of late occurrence of first neurological events have been observed.
For all subsequent cycle starts and reinitiation (e.g. if treatment is interrupted for 4 or more hours), supervision by a healthcare professional or hospitalisation is recommended.
Posology
Relapsed or refractory B-cell precursor ALL
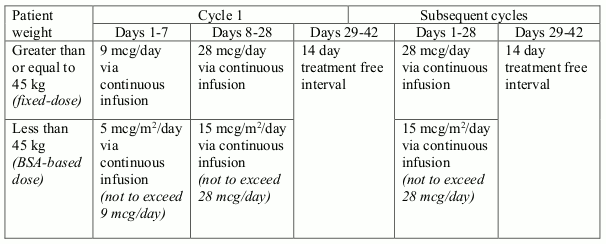
Patients with relapsed or refractory B-cell precursor ALL, may receive 2 cycles of treatment. A single cycle of treatment is 28 days (4 weeks) of continuous infusion. Each cycle of treatment is separated by a 14-day (2 weeks) treatment-free interval.
Patients who have achieved complete remission (CR/CRh*) after 2 treatment cycles may receive up to 3 additional cycles of BLINCYTO consolidation treatment, based on an individual benefits-risks assessment.
Recommended daily dose is by body weight (see table 1). Patients greater than or equal to 45 kg receive a fixed-dose and for patients less than 45 kg, the dose is calculated using the patient's body surface area (BSA).
Table 1. BLINCYTO recommended dosage for relapsed or refractory B-cell precursor ALL:
Premedication and additional medication recommendations
In adult patients, dexamethasone 20 mg intravenous should be administered 1 hour prior to initiation of each cycle of BLINCYTO therapy.
In paediatric patients, dexamethasone 10 mg/m² (not to exceed 20 mg) should be administered orally or intravenously 6 to 12 hours prior to the start of BLINCYTO (cycle 1, day 1). This should be followed by dexamethasone 5 mg/m² orally or intravenously within 30 minutes prior to the start of BLINCYTO (cycle 1, day 1).
Anti-pyretic use (e.g. paracetamol) is recommended to reduce pyrexia during the first 48 hours of each treatment cycle.
Intrathecal chemotherapy prophylaxis is recommended before and during BLINCYTO therapy to prevent central nervous system ALL relapse.
Pre-phase treatment for patients with high tumour burden
For patients with ≥ 50% leukaemic blasts in bone marrow or > 15 000/microlitre peripheral blood leukaemic blast counts treat with dexamethasone (not to exceed 24 mg/day).
MRD positive B-cell precursor ALL
When considering the use of BLINCYTO as a treatment for Philadelphia chromosome-negative MRD positive B-cell precursor ALL, quantifiable MRD should be confirmed in a validated assay with minimum sensitivity of 10-4 (see section 5.1). Clinical testing of MRD, regardless of the choice of technique, should be performed by a qualified laboratory familiar with the technique, following well established technical guidelines.
Patients may receive 1 cycle of induction treatment followed by up to 3 additional cycles of BLINCYTO consolidation treatment. A single cycle of treatment of BLINCYTO induction or consolidation is 28 days (4 weeks) of continuous intravenous infusion followed by a 14-day (2 weeks) treatment-free interval (total 42 days). The majority of patients who respond to blinatumomab achieve a response after 1 cycle (see section 5.1). Therefore, the potential benefit and risks associated with continued therapy in patients who do not show haematological and/or clinical improvement after 1 treatment cycle should be assessed by the treating physician. See table 2 for the recommended daily dose.
Table 2. BLINCYTO recommended dosage for adult patients with MRD-positive B-cell precursor ALL:
| Body weight | Treatment cycle(s) | |
| Days 1-28 | Days 29-42 | |
| Greater than or equal to 45 kg (fixed-dose) | 28 mcg/day | 14-day treatment free interval |
| Less than 45 kg (BSA-based dose) | 15 mcg/m²/day (not to exceed 28 mcg/day) | 14-day treatment-free interval |
Premedication and additional medication recommendations
Prednisone 100 mg intravenously or equivalent (e.g. dexamethasone 16 mg) should be administered 1 hour prior to initiation of each cycle of BLINCYTO therapy.
Anti-pyretic use (e.g. paracetamol) is recommended to reduce pyrexia during the first 48 hours of each treatment cycle.
Intrathecal chemotherapy prophylaxis is recommended before and during BLINCYTO therapy to prevent central nervous system ALL relapse.
B-cell precursor ALL in the consolidation phase
BLINCYTO is administered as a continuous intravenous infusion delivered at a constant flow rate using an infusion pump. A single cycle of treatment is 28 days (4 weeks) of continuous infusion followed by a 14-day (2-week) treatment-free interval. Patients may receive up to 4 cycles of BLINCYTO consolidation treatment.
See table 3 for the recommended daily dose by body weight for adults. Patients weighing greater than or equal to 45 kg receive a fixed-dose, and for patients weighing less than 45 kg, the dose is calculated using the patient's body surface area (BSA).
Table 3. BLINCYTO recommended dosage for B-cell precursor ALL for Adults in the consolidation phase:
| Body weight | Consolidation cycles (Cycles 1-4) | |
| Days 1-28 | Days 29-42 | |
| Greater than or equal to 45 kg (fixed-dose) | 28 mcg/day | 14 day treatment-free interval |
| Less than 45 kg (BSA-based dose) | 15 mcg/m²/day (not to exceed 28 mcg/day) | 14 day treatment-free interval |
High-risk first relapsed B-cell precursor ALL
Paediatric patients with high-risk first relapsed B-cell precursor ALL may receive 1 cycle of BLINCYTO treatment after induction and 2 blocks of consolidation chemotherapy. A single cycle of treatment is 28 days (4 weeks) of continuous infusion. See table 4 for the recommended daily dose by body weight for paediatric patients.
Table 4. BLINCYTO recommended dosage for paediatric patients with high-risk first relapsed B-cell precursor ALL post-induction chemotherapy:
| One consolidation cycle | Body weight greater than or equal to 45 kg (fixed-dose) | Body weight less than 45 kg (BSA-based dose) |
| Days 1-28 | 28 mcg/day | 15 mcg/m²/day (not to exceed 28 mcg/day) |
Premedication and additional medication recommendations
In adult patients, dexamethasone 20 mg intravenous should be administered within 1 hour prior to initiation of each cycle of BLINCYTO therapy.
In paediatric patients, dexamethasone 5 mg/m² (not to exceed 20 mg) should be administered prior to the first dose of BLINCYTO in the first cycle and when restarting an infusion after an interruption of 4 or more hours in the first cycle.
Intrathecal chemotherapy prophylaxis is recommended before and during BLINCYTO therapy to prevent central nervous system ALL relapse.
Dose adjustments
Consideration to discontinue BLINCYTO temporarily or permanently as appropriate should be made in the case of the following severe (grade 3) or life-threatening (grade 4) toxicities (see section 4.4): cytokine release syndrome, tumour lysis syndrome, neurological toxicity, elevated liver enzymes and any other clinically relevant toxicities.
If the interruption of treatment after an adverse reaction is no longer than 7 days, continue the same cycle to a total of 28 days of infusion inclusive of days before and after the interruption in that cycle. If an interruption due to an adverse reaction is longer than 7 days, start a new cycle. If the toxicity takes more than 14 days to resolve, discontinue BLINCYTO permanently, except if described differently in the table 5 below.
Table 5. BLINCYTO dose adjustments:
| Toxicity | Grade* | Action for patients weighing greater than or equal to 45 kg | Action for patients weighing less than 45 kg | |||
| Cytokine release syndrome, tumour lysis syndrome | Grade 3 | Interrupt BLINCYTO until resolved, then restart BLINCYTO at 9 mcg/day. Escalate to 28 mcg/day after 7 days if the toxicity does not recur. | Interrupt BLINCYTO until resolved, then restart BLINCYTO at 5 mcg/m²/day. Escalate to 15 mcg/m²/day after 7 days if the toxicity does not recur. | |||
| Grade 4 | Discontinue BLINCYTO permanently. | Discontinue BLINCYTO permanently.\ | Neurological toxicity | Convulsion | Discontinue BLINCYTO permanently if more than one convulsion occurs. | Discontinue BLINCYTO permanently if more than one convulsion occurs. |
| Grade 3 | Interrupt BLINCYTO until no more than grade 1 (mild) and for at least 3 days, then restart BLINCYTO at 9 mcg/day. Escalate to 28 mcg/day after 7 days if the toxicity does not recur. For reinitiation, premedicate with a 24 mg dose of dexamethasone. Then reduce dexamethasone step-wise over 4 days. If the toxicity occurred at 9 mcg/day, or if the toxicity takes more than 7 days to resolve, discontinue BLINCYTO permanently. | Interrupt BLINCYTO until no more than grade 1 (mild) and for at least 3 days, then restart BLINCYTO at 5 mcg/m²/day. Escalate to 15 mcg/m²/day after 7 days if the toxicity does not recur. If the toxicity occurred at 5 mcg/m²/day, or if the toxicity takes more than 7 days to resolve, discontinue BLINCYTO permanently. | ||||
| Grade 4 | Discontinue BLINCYTO permanently. | Discontinue BLINCYTO permanently. | ||||
| Elevated liver enzymes | Grade 3 | If clinically relevant, interrupt BLINCYTO until no more than grade 1 (mild), then restart BLINCYTO at 9 mcg/day. Escalate to 28 mcg/day after 7 days if the toxicity does not recur. | If clinically relevant, interrupt BLINCYTO until no more than grade 1 (mild), then restart BLINCYTO at 5 mcg/m²/day. Escalate to 15 mcg/m²/day after 7 days if the toxicity does not recur. | |||
| Grade 4 | Consider discontinuing BLINCYTO permanently. | Consider discontinuing BLINCYTO permanently. | ||||
| Other clinically relevant (as determined by treating physician) adverse reactions | Grade 3 | Interrupt BLINCYTO until no more than grade 1 (mild), then restart BLINCYTO at 9 mcg/day. Escalate to 28 mcg/day after 7 days if the toxicity does not recur. | Interrupt BLINCYTO until no more than grade 1 (mild), then restart BLINCYTO at 5 mcg/m²/day. Escalate to 15 mcg/m²/day after 7 days if the toxicity does not recur. | |||
| Grade 4 | Consider discontinuing BLINCYTO permanently. | Consider discontinuing BLINCYTO permanently. |
* Based on the NCI Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Grade 3 is severe, and grade 4 is life-threatening.
Special populations
Elderly
No dose adjustment is necessary in elderly patients (≥65 years of age), see section 5.1. There is limited experience with BLINCYTO in patients ≥75 years of age.
Renal impairment
Based on pharmacokinetic analyses, dose adjustment is not necessary in patients with mild to moderate renal dysfunction (see section 5.2). The safety and efficacy of BLINCYTO have not been studied in patients with severe renal impairment.
Hepatic impairment
Based on pharmacokinetic analyses, no effect of baseline liver function on blinatumomab exposure is expected and adjustment of the initial dose is not necessary (see section 5.2). The safety and efficacy of BLINCYTO have not been studied in patients with severe hepatic impairment.
Paediatric population
There is limited experience with BLINCYTO in children < 1 year of age. Currently available data in children are described in sections 4.8 and 5.1.
Method of administration
BLINCYTO is for intravenous use.
For instructions on the handling and preparation of the medicinal product before administration, see section 6.6.
Administer BLINCYTO as a continuous intravenous infusion delivered at a constant flow rate using an infusion pump over a period of up to 96 hours. The pump should be programmable, lockable, non-elastomeric, and have an alarm.
The starting volume (270 mL) is more than the volume administered to the patient (240 mL) to account for the priming of the intravenous tubing and to ensure that the patient will receive the full dose of BLINCYTO.
Infuse prepared BLINCYTO final infusion solution according to the instructions on the pharmacy label on the prepared bag at one of the following constant infusion rates:
- Infusion rate of 10 mL/h for a duration of 24 hours
- Infusion rate of 5 mL/h for a duration of 48 hours
- Infusion rate of 3.3 mL/h for a duration of 72 hours
- Infusion rate of 2.5 mL/h for a duration of 96 hours
Administer prepared BLINCYTO final infusion solution using intravenous tubing that contains a sterile, non-pyrogenic, low protein-binding 0.2 micrometre in-line filter.
Important note: Do not flush the BLINCYTO infusion line, especially when changing infusion bags. Flushing when changing bags or at completion of infusion can result in excess dosage and complications thereof. When administering via a multi-lumen venous catheter, BLINCYTO should be infused through a dedicated lumen.
The choice of the infusion duration should be made by the treating physician considering the frequency of the infusion bag changes and the weight of the patient. The target therapeutic dose of BLINCYTO delivered does not change.
Change of infusion bag
The infusion bag must be changed at least every 96 hours by a healthcare professional for sterility reasons.
4.9. Overdose
Overdoses have been observed including one patient who received 133-fold the recommended therapeutic dose of BLINCYTO delivered over a short duration. Overdoses resulted in adverse reactions which were consistent with the reactions observed at the recommended therapeutic dose and included fever, tremors, and headache. In the event of overdose, the infusion should be temporarily interrupted and patients should be monitored. Reinitiation of BLINCYTO at the correct therapeutic dose should be considered when all toxicities have resolved and no earlier than 12 hours after interruption of the infusion (see section 4.2).
6.3. Shelf life
Unopened vials:
5 years.
Reconstituted solution:
Chemical and physical in-use stability has been demonstrated for 24 hours at 2°C-8°C or 4 hours at or below 27°C.
From a microbiological point of view, unless the method of reconstituting precludes the risks of microbial contamination, the reconstituted solution should be diluted immediately. If not diluted immediately, in-use storage times and conditions are the responsibility of the user.
Diluted solution (prepared infusion bag):
Chemical and physical in-use stability has been demonstrated for 10 days at 2°C-8°C or 96 hours at or below 27°C.
From a microbiological point of view, the prepared infusion bags should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2°C-8°C, unless dilution has taken place in controlled and validated aseptic conditions.
6.4. Special precautions for storage
Store and transport refrigerated (2°C-8°C).
Do not freeze.
Store the vials in the original package in order to protect from light.
For storage conditions after reconstitution and dilution of the medicinal product, see section 6.3.
6.5. Nature and contents of container
Each BLINCYTO pack contains 1 vial of powder for concentrate for solution for infusion and 1 vial of solution (stabiliser):
- 38.5 micrograms blinatumomab powder in a vial (type I glass) with a stopper (elastomeric rubber), seal (aluminium) and a flip off cap, and
- 10 mL solution in a vial (type I glass) with a stopper (elastomeric rubber), seal (aluminium) and a flip off cap.
6.6. Special precautions for disposal and other handling
Aseptic preparation:
Aseptic handling must be ensured when preparing the infusion. Preparation of BLINCYTO should be:
- performed under aseptic conditions by trained personnel in accordance with good practice rules especially with respect to the aseptic preparation of parenteral products.
- prepared in a laminar flow hood or biological safety cabinet using standard precautions for the safe handling of intravenous agents.
It is very important that the instructions for preparation and administration provided in this section are strictly followed to minimise medication errors (including underdose and overdose).
Other instructions:
- BLINCYTO is compatible with polyolefin, PVC non-di-ethylhexylphthalate (non-DEHP), or ethyl vinyl acetate (EVA) infusion bags/pump cassettes.
- At the end of infusion, any unused medicinal product or waste material should be disposed of in accordance with local requirements.
Preparation of the solution for infusion:
These supplies are also required, but not included in the package:
- Sterile single-use disposable syringes
- 21-23 gauge needle(s) (recommended)
- Water for injections
- Infusion bag with 250 mL sodium chloride 9 mg/mL (0.9%) solution for injection;
- To minimise the number of aseptic transfers, use a 250 mL pre-filled infusion bag. BLINCYTO dose calculations are based on a usual overfill volume of 265 to 275 mL sodium chloride 9 mg/mL (0.9%) solution for injection.
- Use only polyolefin, PVC non-di-ethylhexylphthalate (non-DEHP), or ethyl vinyl acetate (EVA) infusion bags/pump cassettes.
- Polyolefin, PVC non-DEHP, or EVA intravenous tubing with a sterile, non-pyrogenic, low protein-binding 0.2 micrometre in-line filter.
- Ensure that the tubing is compatible with the infusion pump.
Reconstitute BLINCYTO with water for injections. Do not reconstitute BLINCYTO vials with the solution (stabiliser).
To prime the intravenous tubing, use only the solution in the bag containing the FINAL prepared BLINCYTO solution for infusion. Do not prime with sodium chloride 9 mg/mL (0.9%) solution for injection.
Reconstitution of BLINCYTO
1. Determine the number of BLINCYTO vials needed for a dose and infusion duration.
2. Using a syringe, reconstitute each vial of BLINCYTO powder for concentrate using 3 mL of water for injections. Direct the water along the walls of the BLINCYTO vial and not directly on the lyophilised powder.
- Do not reconstitute BLINCYTO powder for concentrate with the solution (stabiliser).
- The addition of water for injections to the powder for concentrate results in a total volume of 3.08 mL for a final BLINCYTO concentration of 12.5 mcg/mL.
3. Gently swirl contents to avoid excess foaming.
- Do not shake.
4. Visually inspect the reconstituted solution for particulate matter and discolouration during reconstitution and prior to infusion. The resulting solution should be clear to slightly opalescent, colourless-to-slightly yellow.
- Do not use if the solution is cloudy or has precipitated.
Preparation of BLINCYTO infusion bag
Verify the prescribed dose and infusion duration for each BLINCYTO infusion bag. To minimise errors, use the specific volumes described in tables 13 and 14 to prepare the BLINCYTO infusion bag.
- Table 18 for patients weighing greater than or equal to 45 kg
- Table 19 for patients weighing less than 45 kg
1. Use an infusion bag pre-filled with 250 mL sodium chloride 9 mg/mL (0.9%) solution for injection that usually contains a total volume of 265 to 275 mL.
2. To coat the infusion bag, using a syringe, aseptically transfer 5.5 mL of the solution (stabiliser) to the infusion bag. Gently mix the contents of the bag to avoid foaming. Discard the remaining solution (stabiliser).
3. Using a syringe, aseptically transfer the required volume of reconstituted BLINCYTO solution into the infusion bag containing sodium chloride 9 mg/mL (0.9%) solution for injection and the solution (stabiliser). Gently mix the contents of the bag to avoid foaming.
- Refer to table 18 for patients weighing greater than or equal to 45 kg for the specific volume of reconstituted BLINCYTO.
- Refer to table 19 for patients weighing less than 45 kg (dose based on BSA) for the specific volume of reconstituted BLINCYTO.
- Discard the vial containing any unused BLINCYTO reconstituted solution.
4. Under aseptic conditions, attach the intravenous tubing to the infusion bag with the sterile 0.2 micron in-line filter. Ensure that the intravenous tubing is compatible with the infusion pump.
5. Remove air from the infusion bag. This is particularly important for use with an ambulatory infusion pump.
6. Prime the intravenous infusion line only with the solution in the bag containing the FINAL prepared BLINCYTO solution for infusion.
7. Store refrigerated at 2°C-8°C if not used immediately.
Table 18. For patients weighing greater than or equal to 45 kg: volumes of sodium chloride 9 mg/mL (0.9%) solution for injection, solution (stabiliser), and reconstituted BLINCYTO to add to infusion bag:
| Sodium chloride 9 mg/mL (0.9%) solution for injection (starting volume) | 250 ml (usual overfill volume of 265 to 275 mL) | |||
| Solution (stabiliser) (fixed volume for 24, 48, 72, and 96-hour infusion durations) | 5.5 ml | |||
| Infusion uration | Dose | Infusion rate | Reconstituted BLINCYTO | |
| Volume | Vials | |||
| 24 hours | 9 mcg/day | 10 mL/hour | 0.83 ml | 1 |
| 28 mcg/day | 10 mL/hour | 2.6 ml | 1 | |
| 48 hours | 9 mcg/day | 5 mL/hour | 1.7 ml | 1 |
| 28 mcg/day | 5 mL/hour | 5.2 ml | 2 | |
| 72 hours | 9 mcg/day | 3.3 mL/hour | 2.5 ml | 1 |
| 28 mcg/day | 3.3 mL/hour | 8 ml | 3 | |
| 96 hours | 9 mcg/day | 2.5 mL/hour | 3.3 ml | 2 |
| 28 mcg/day | 2.5 mL/hour | 10.7 ml | 4 | |
Table 19. For patients weighing less than 45 kg: volumes of sodium chloride 9 mg/mL (0.9%) solution for injection, solution (stabiliser), and reconstituted BLINCYTO to add to infusion bag:
| Sodium chloride 9 mg/mL (0.9%) solution for injection (starting volume) | 250 ml (usual overfill volume of 265 to 275 mL) | ||||
| Solution (stabiliser) (fixed volume for 24, 48, 72, and 96-hour infusion durations) | 5.5 ml | ||||
| Infusion uration | Dose | Infusion rate | BSA (m²)* | Reconstituted BLINCYTO | |
| Volume | Vials | ||||
| 24 hours | 5 mcg/m²/ day | 10 mL/hour | 1.5 – 1.59 | 0.7 ml | 1 |
| 1.4 – 1.49 | 0.66 ml | 1 | |||
| 1.3 – 1.39 | 0.61 ml | 1 | |||
| 1.2 – 1.29 | 0.56 ml | 1 | |||
| 1.1 – 1.19 | 0.52 ml | 1 | |||
| 1 – 1.09 | 0.47 ml | 1 | |||
| 0.9 – 0.99 | 0.43 ml | 1 | |||
| 0.8 – 0.89 | 0.38 ml | 1 | |||
| 0.7 – 0.79 | 0.33 ml | 1 | |||
| 0.6 – 0.69 | 0.29 ml | 1 | |||
| 0.5 – 0.59 | 0.24 ml | 1 | |||
| 0.4 – 0.49 | 0.2 ml | 1 | |||
| 24 hours | 15 mcg/m²/ day | 10 mL/hour | 1.5 – 1.59 | 2.1 ml | 1 |
| 1.4 – 1.49 | 2 ml | 1 | |||
| 1.3 – 1.39 | 1.8 ml | 1 | |||
| 1.2 – 1.29 | 1.7 ml | 1 | |||
| 1.1 – 1.19 | 1.6 ml | 1 | |||
| 1 – 1.09 | 1.4 ml | 1 | |||
| 0.9 – 0.99 | 1.3 ml | 1 | |||
| 0.8 – 0.89 | 1.1 ml | 1 | |||
| 0.7 – 0.79 | 1 ml | 1 | |||
| 0.6 – 0.69 | 0.86 ml | 1 | |||
| 0.5 – 0.59 | 0.72 ml | 1 | |||
| 0.4 – 0.49 | 0.59 ml | 1 | |||
| 48 hours | 5 mcg/m²/ day | 5 mL/hour | 1.5 – 1.59 | 1.4 ml | 1 |
| 1.4 – 1.49 | 1.3 ml | 1 | |||
| 1.3 – 1.39 | 1.2 ml | 1 | |||
| 1.2 – 1.29 | 1.1 ml | 1 | |||
| 1.1 – 1.19 | 1 ml | 1 | |||
| 1 – 1.09 | 0.94 ml | 1 | |||
| 0.9 – 0.99 | 0.85 ml | 1 | |||
| 0.8 – 0.89 | 0.76 ml | 1 | |||
| 0.7 – 0.79 | 0.67 ml | 1 | |||
| 0.6 – 0.69 | 0.57 ml | 1 | |||
| 0.5 – 0.59 | 0.48 ml | 1 | |||
| 0.4 – 0.49 | 0.39 ml | 1 | |||
| 48 hours | 15 mcg/m²/ day | 5 mL/hour | 1.5 – 1.59 | 4.2 ml | 2 |
| 1.4 – 1.49 | 3.9 ml | 2 | |||
| 1.3 – 1.39 | 3.7 ml | 2 | |||
| 1.2 – 1.29 | 3.4 ml | 2 | |||
| 1.1 – 1.19 | 3.1 ml | 2 | |||
| 1 – 1.09 | 2.8 ml | 1 | |||
| 0.9 – 0.99 | 2.6 ml | 1 | |||
| 0.8 – 0.89 | 2.3 ml | 1 | |||
| 0.7 – 0.79 | 2 ml | 1 | |||
| 0.6 – 0.69 | 1.7 ml | 1 | |||
| 0.5 – 0.59 | 1.4 ml | 1 | |||
| 0.4 – 0.49 | 1.2 ml | 1 | |||
| 72 hours | 5 mcg/m²/ day | 3.3 mL/hour | 1.5 – 1.59 | 2.1 ml | 1 |
| 1.4 – 1.49 | 2 ml | 1 | |||
| 1.3 – 1.39 | 1.8 ml | 1 | |||
| 1.2 – 1.29 | 1.7 ml | 1 | |||
| 1.1 – 1.19 | 1.6 ml | 1 | |||
| 1 – 1.09 | 1.4 ml | 1 | |||
| 0.9 – 0.99 | 1.3 ml | 1 | |||
| 0.8 – 0.89 | 1.1 ml | 1 | |||
| 0.7 – 0.79 | 1 ml | 1 | |||
| 0.6 – 0.69 | 0.86 ml | 1 | |||
| 0.5 – 0.59 | 0.72 ml | 1 | |||
| 0.4 – 0.49 | 0.59 ml | 1 | |||
| 72 hours | 15 mcg/m²/ day | 3.3 mL/hour | 1.5 – 1.59 | 6.3 ml | 3 |
| 1.4 – 1.49 | 5.9 ml | 3 | |||
| 1.3 – 1.39 | 5.5 ml | 2 | |||
| 1.2 – 1.29 | 5.1 ml | 2 | |||
| 1.1 – 1.19 | 4.7 ml | 2 | |||
| 1 – 1.09 | 4.2 ml | 2 | |||
| 0.9 – 0.99 | 3.8 ml | 2 | |||
| 0.8 – 0.89 | 3.4 ml | 2 | |||
| 0.7 – 0.79 | 3 ml | 2 | |||
| 0.6 – 0.69 | 2.6 ml | 1 | |||
| 0.5 – 0.59 | 2.2 ml | 1 | |||
| 0.4 – 0.49 | 1.8 ml | 1 | |||
| 96 hours | 5 mcg/m²/ day | 2.5 mL/hour | 1.5 – 1.59 | 2.8 ml | 1 |
| 1.4 – 1.49 | 2.6 ml | 1 | |||
| 1.3 – 1.39 | 2.4 ml | 1 | |||
| 1.2 – 1.29 | 2.3 ml | 1 | |||
| 1.1 – 1.19 | 2.1 ml | 1 | |||
| 1 – 1.09 | 1.9 ml | 1 | |||
| 0.9 – 0.99 | 1.7 ml | 1 | |||
| 0.8 – 0.89 | 1.5 ml | 1 | |||
| 0.7 – 0.79 | 1.3 ml | 1 | |||
| 0.6 – 0.69 | 1.2 ml | 1 | |||
| 0.5 – 0.59 | 0.97 ml | 1 | |||
| 0.4 – 0.49 | 0.78 ml | 1 | |||
| 96 hours | 15 mcg/m²/ day | 2.5 mL/hour | 1.5 – 1.59 | 8.4 ml | 3 |
| 1.4 – 1.49 | 7.9 ml | 3 | |||
| 1.3 – 1.39 | 7.3 ml | 3 | |||
| 1.2 – 1.29 | 6.8 ml | 3 | |||
| 1.1 – 1.19 | 6.2 ml | 3 | |||
| 1 – 1.09 | 5.7 ml | 3 | |||
| 0.9 – 0.99 | 5.1 ml | 2 | |||
| 0.8 – 0.89 | 4.6 ml | 2 | |||
| 0.7 – 0.79 | 4 ml | 2 | |||
| 0.6 – 0.69 | 3.4 ml | 2 | |||
| 0.5 – 0.59 | 2.9 ml | 2 | |||
| 0.4 – 0.49 | 2.3 ml | 1 | |||
BSA = body surface area
* The safety of the administration of BLINCYTO for BSA of less than 0.4 m² has not been established.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.