BOTOX Powder for solution for injection Ref.[6581] Active ingredients: Botulinum toxin type A
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2022 Publisher: AbbVie Ltd., Maidenhead, SL6 4UB, UK
Therapeutic indications
BOTOX is indicated for:
Neurologic disorders:
- treatment of focal spasticity, including:
- elbow, wrist and hand in paediatric cerebral palsy patients, two years of age or older as an adjunct to rehabilitative therapy
- ankle and foot in ambulant paediatric cerebral palsy patients, two years of age or older as an adjunct to rehabilitative therapy
- wrist and hand disability due to upper limb spasticity associated with stroke in adults
- ankle and foot disability due to lower limb spasticity associated with stroke in adults
- symptomatic relief of blepharospasm, hemifacial spasm and idiopathic cervical dystonia (spasmodic torticollis)
- prophylaxis of headaches in adults with chronic migraine (headaches on at least 15 days per month of which at least 8 days are with migraine)
Bladder disorders:
- management of bladder dysfunctions in adult patients who are not adequately managed with anticholinergics
- overactive bladder with symptoms of urinary incontinence, urgency and frequency
- neurogenic detrusor overactivity with urinary incontinence due to subcervical spinal cord injury (traumatic or non-traumatic), or multiple sclerosis
Skin and skin appendage disorders:
- management of severe hyperhidrosis of the axillae, which does not respond to topical treatment with antiperspirants or antihidrotics
- temporary improvement in the appearance of:
- moderate to severe vertical lines between the eyebrows seen at maximum frown (glabellar lines) and/or,
- moderate to severe lateral canthal lines (crow’s feet lines) seen at maximum smile and/or,
- moderate to severe forehead lines seen at maximum eyebrow elevation
when the severity of the facial lines has an important psychological impact in adult patients.
Posology and method of administration
Posology
Botulinum toxin units are not interchangeable from one product to another. Doses recommended in Allergan Units are different from other botulinum toxin preparations.
Elderly patients
Dosages for elderly patients are the same as for younger adults. Initial dosing should begin at the lowest recommended dose for the specific indication. Elderly patients with significant medical history and concomitant medications should be treated with caution.
There is limited data in patients older than 65 years managed with BOTOX for urinary incontinence with neurogenic detrusor overactivity, ankle and foot disability due to lower limb spasticity associated with stroke, and for facial lines (see section 5.1).
Paediatric population
The safety and efficacy of BOTOX in indications other than those described for the paediatric population in section 4.1 have not been established. No recommendation on posology can be made for indications other than paediatric focal spasticity associated with cerebral palsy. Currently available data per indication are described in section 4.2, 4.4, 4.8 and 5.1, as shown in the table below.
BOTOX should only be administered by appropriately qualified healthcare practitioners who are experienced in the assessment and treatment of paediatric focal spasticity and as part of a structured program of rehabilitation.
| Focal spasticity in paediatric patients | 2 years (see section 4.2, 4.4 and 4.8) |
| Blepharospasm/Hemifacial spasm/ Idiopathic Cervical dystonia | 12 years (see section 4.4 and 4.8) |
| Primary hyperhidrosis of the axillae | 12 years (limited experience in adolescents between 12 and 17 years, see sections 4.4, 4.8 and 5.1) |
Method of Administration
BOTOX should only be administered by physicians with appropriate qualifications and expertise in the treatment and the use of the required equipment.
This product is for single use only and any unused solution should be discarded. The most appropriate vial size should be selected for the indication.
An injection volume of approximately 0.1 ml is recommended. A decrease or increase in the BOTOX dose is possible by administering a smaller or larger injection volume. The smaller the injection volume the less discomfort and less spread of toxin in the injected muscle occurs. This is of benefit in reducing effects on nearby muscles when small muscle groups are being injected.
For instructions on reconstitution of the powder for solution for injection, handling and disposal of vials please refer to section 6.6.
Refer to specific guidance for each indication described below.
Generally valid optimum dose levels and number of injection sites per muscle have not been established for all indications. In these cases, individual treatment regimens should therefore be drawn up by the physician. Optimum dose levels should be determined by titration but the recommended maximum dose should not be exceeded.
NEUROLOGIC DISORDERS
Focal spasticity of the upper limb in paediatric patients
Recommended needle: Appropriately sized sterile needle. Needle length should be determined based on muscle location and depth.
Administration guidance: Localisation of the involved muscles with techniques such as needle electromyographic guidance, nerve stimulation, or ultrasound is recommended. Prior to injection, local anaesthesia or local anaesthesia in combination with minimal or moderate sedation may be used, per local site practice. The safety and efficacy of BOTOX in the treatment of paediatric spasticity has not been evaluated under general anaesthesia or deep sedation/analgesia.
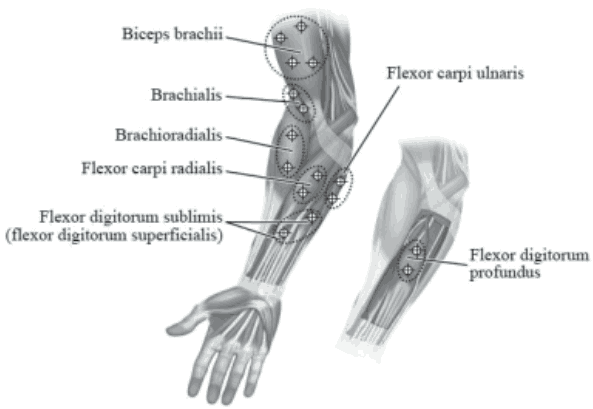
The following diagram indicates the injection sites for paediatric upper limb spasticity:
Recommended dose: The recommended dose for treating paediatric upper limb spasticity is 3 Units/kg to 6 Units/kg body weight divided among the affected muscles.
BOTOX Dosing by Muscle for Paediatric Upper Limb Spasticity:
| Muscles Injected | BOTOX 3 Units/kg (maximum Units per muscle) | BOTOX 6 Units/kg (maximum Units per muscle) | Number of Injection Sites |
|---|---|---|---|
| Elbow Flexor Muscles | |||
| Biceps | 1.5 Units/kg (50 Units) | 3 Units/kg (100 Units) | 4 |
| Brachialis | 1 Unit/kg (30 Units) | 2 Units/kg (60 Units) | 2 |
| Brachioradialis | 0.5 Units/kg (20 Units) | 1 Unit/kg (40 Units) | 2 |
| Wrist Muscles | |||
| Flexor carpi radialis | 1 Unit/kg (25 Units) | 2 Units/kg (50 Units) | 2 |
| Flexor carpi ulnaris | 1 Unit/kg (25 Units) | 2 Units/kg (50 Units) | 2 |
| Finger Muscles | |||
| Flexor digitorum profundus | 0.5 Units/kg (25 Units) | 1 Unit/kg (50 Units) | 2 |
| Flexor digitorum sublimis | 0.5 Units/kg (25 Units) | 1 Unit/kg (50 Units) | 2 |
Maximum dose: The total dose of BOTOX administered per treatment session in the upper limb should not exceed 6 Units/kg body weight or 200 Units, whichever is lower. If it is deemed appropriate by the treating healthcare practitioner, the patient should be considered for re-injection when the clinical effect of the previous injection has diminished, no sooner than 12 weeks after the previous injection. When treating the upper and lower limbs in combination, the total dose should not exceed the lower of 10 Units/kg body weight or 340 Units, in a 12-week interval.
Additional information: Treatment with BOTOX is not intended to substitute for usual standard of care rehabilitation regimens. Clinical improvement generally occurs within the first two weeks after injection. Repeat treatment should be administered when the clinical effect of a previous injection diminishes but not more frequently than every 12 weeks.
Focal spasticity of the lower limb in paediatric patients
Recommended needle: Appropriately sized sterile needle. Needle length should be determined based on muscle location and depth.
Administration guidance: Localisation of the involved muscles with techniques such as needle electromyographic guidance, nerve stimulation, or ultrasound is recommended. Prior to injection, local anaesthesia or local anaesthesia in combination with minimal or moderate sedation may be used, per local site practice. The safety and efficacy of BOTOX in the treatment of paediatric spasticity has not been evaluated under general anaesthesia or deep sedation/analgesia.
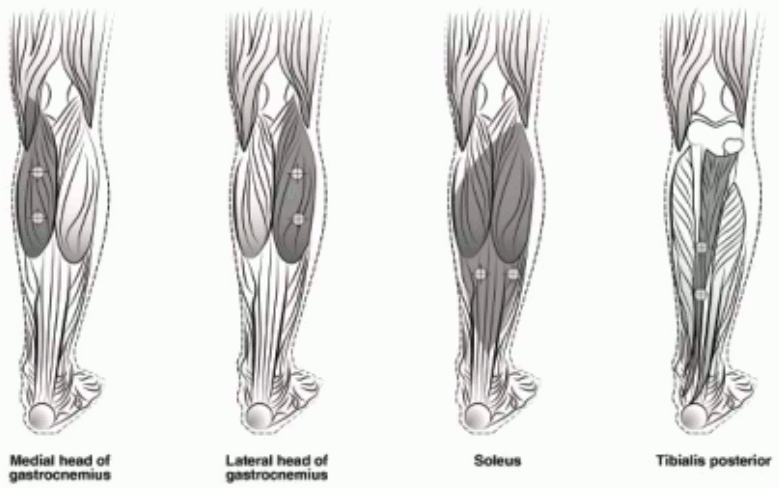
The following diagram indicates the injection sites for paediatric lower limb spasticity:
Recommended dose: The recommended dose for paediatric lower limb spasticity is 4 Units/kg to 8 Units/kg body weight divided among the affected muscles.
BOTOX Dosing by Muscle for Paediatric Lower Limb Spasticity:
| Muscles Injected | BOTOX 4 Units/kg (maximum Units per muscle) | BOTOX 8 Units/kg (maximum Units per muscle) | Number of Injection Sites |
|---|---|---|---|
| Gastrocnemius medial head | 1 Unit/kg (37.5 Units) | 2 Units/kg (75 Units) | 2 |
| Gastrocnemius lateral head | 1 Unit/kg (37.5 Units) | 2 Units/kg (75 Units) | 2 |
| Soleus | 1 Unit/kg (37.5 Units) | 2 Units/kg (75 Units) | 2 |
| Tibialis Posterior | 1 Unit/kg (37.5 Units) | 2 Units/kg (75 Units) | 2 |
Maximum dose: The total dose of BOTOX administered per treatment session in the lower limb should not exceed 8 Units/kg body weight or 300 Units, whichever is lower. If it is deemed appropriate by the treating healthcare practitioner, the patient should be considered for re-injection when the clinical effect of the previous injection has diminished, no sooner than 12 weeks after the previous injection. When treating both lower limbs or the upper and lower limbs in combination, the total dose should not exceed the lower of 10 Units/kg body weight or 340 Units, in a 12 week interval.
Additional information: Treatment with BOTOX is not intended to substitute for usual standard of care rehabilitation regimens. Clinical improvement generally occurs within the first two weeks after injection. Repeat treatment should be administered when the clinical effect of a previous injection diminishes but not more frequently than every 12 weeks.
Focal upper limb spasticity associated with stroke in adults
Recommended needle: Sterile 25, 27 or 30 gauge needle. Needle length should be determined based on muscle location and depth.
Administration guidance: Localisation of the involved muscles with techniques such as electromyographic guidance, nerve stimulation, or ultrasound is recommended. Multiple injection sites may allow BOTOX to have more uniform contact with the innervation areas of the muscle and are especially useful in larger muscles.
Recommended dose: The exact dosage and number of injection sites may be tailored to the individual based on the size, number and location of muscles involved, the severity of spasticity, the presence of local muscle weakness, and the patient response to previous treatment.
The following doses are recommended:
| Muscle | Total Dosage; Number of Sites |
|---|---|
| Flexor digitorum profundus | 15-50 Units; 1-2 sites |
| Flexor digitorum sublimis | 15-50 Units; 1-2 sites |
| Flexor carpi radialis | 15-60 Units; 1-2 sites |
| Flexor carpi ulnaris | 10-50 Units; 1-2 sites |
| Adductor Pollicis | 20 Units; 1-2 sites |
| Flexor Pollicis Longus | 20 Units; 1-2 sites |
Maximum dose: Between 200 and 240 Units divided among selected muscles.
Additional information: If it is deemed appropriate by the treating physician, the patient should be considered for re-injection when the clinical effect of the previous injection has diminished. Re-injections should occur no sooner than 12 weeks after the previous injection. The degree and pattern of muscle spasticity at the time of re-injection may necessitate alterations in the dose of BOTOX and muscles to be injected. The lowest effective dose should be used.
Focal lower limb spasticity associated with stroke in adults
Recommended needle: Sterile 25, 27 or 30 gauge needle. Needle length should be determined based on muscle location and depth.
Administration guidance: Localisation of the involved muscles with techniques such as electromyographic guidance, nerve stimulation, or ultrasound is recommended. Multiple injection sites may allow BOTOX to have more uniform contact with the innervation areas of the muscle and are especially useful in larger muscles.
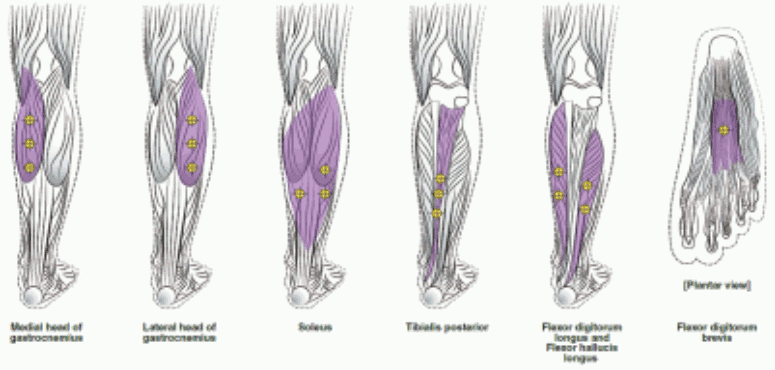
The following diagrams indicate the injection sites for adult lower limb spasticity:
Recommended dose: 300 Units to 400 Units divided among up to 6 muscles, as listed in the following table.
| Muscle | Recommended Dose |
|---|---|
| Total Dosage; Number of Sites | |
| Gastrocnemius Medial head Lateral head | 75 Units; 3 sites 75 Units; 3 sites |
| Soleus | 75 Units; 3 sites |
| Tibialis Posterior | 75 Units; 3 sites |
| Flexor hallucis longus | 50 Units; 2 sites |
| Flexor digitorum longus | 50 Units; 2 sites |
| Flexor digitorum brevis | 25 Units; 1 site |
Maximum dose: 400 Units in total.
Additional information: If it is deemed appropriate by the treating physician, the patient should be considered for re-injection when the clinical effect of the previous injection has diminished, no sooner than 12 weeks after the previous injection.
Blepharospasm/hemifacial spasm
Recommended needle: Sterile, 27-30 gauge/0.40-0.30 mm needle.
Administrative guidance: Electromyographic guidance is not necessary.
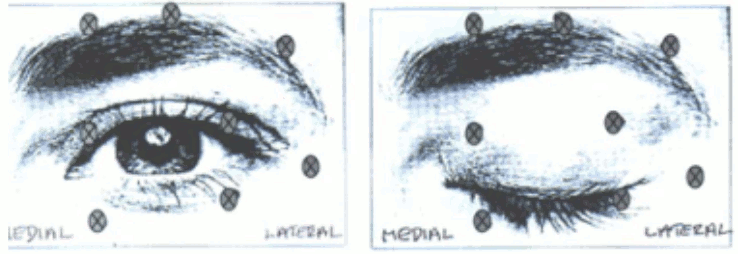
Recommended dose: The initial recommended dose is 1.25-2.5 Units (0.05-0.1 ml volume at each site) injected into the medial and lateral orbicularis oculi of the upper lid and the lateral orbicularis oculi of the lower lid. Additional sites in the brow area, the lateral orbicularis and in the upper facial area may also be injected if spasms here interfere with vision.
The following diagrams indicate the possible injection sites:
Maximum dose: The initial dose should not exceed 25 Units per eye. In the management of blepharospasm total dosing should not exceed 100 Units in total every 12 weeks.
Additional information: Avoiding injection near levator palpebrae superioris may reduce the complication of ptosis. Avoiding medial lower lid injections, and thereby reducing diffusion into the inferior oblique, may reduce the complication of diplopia.
In general, the initial effect of the injections is seen within three days and reaches a peak at one to two weeks post-treatment. Each treatment lasts approximately three months, following which the procedure can be repeated indefinitely. Normally no additional benefit is conferred by treating more frequently than every three months.
At repeat treatment sessions, the dose may be increased up to two-fold if the response from the initial treatment is considered insufficient – usually defined as an effect that does not last longer than two months. However, there appears to be little benefit obtainable from injecting more than 5 Units per site.
Patients with hemifacial spasm or VIIth nerve disorders should be treated as for unilateral blepharospasm, with other affected facial muscles being injected as needed. Electromyographic control may be necessary to identify affected small circumoral muscles.
Cervical dystonia
Recommended needle: A 25, 27 or 30 gauge/0.50-0.30 mm needle may be used for superficial muscles, and a 22 gauge needle may be used for deeper musculature.
Administrative guidance: The treatment of cervical dystonia typically may include injection of BOTOX into the sternocleidomastoid, levator scapulae, scalene, splenius capitis, semispinalis, longissimus and/or the trapezius muscle(s). This list is not exhaustive as any of the muscles responsible for controlling head position may be involved and therefore require treatment. The muscle mass and the degree of hypertrophy are factors to be taken into consideration when selecting the appropriate dose. Muscle activation patterns can change spontaneously in cervical dystonia without a change in the clinical presentation of dystonia.
In case of any difficulty in isolating the individual muscles, injections should be made under electromyographic assistance.
Multiple injection sites allow BOTOX to have more uniform contact with the innervation areas of the dystonic muscle and are especially useful in larger muscles. The optimal number of injection sites is dependent upon the size of the muscle to be chemically denervated.
Recommended dose: Dosing must be tailored to the individual patient based on the patient’s head and neck position, location of pain, muscle hypertrophy, patient’s body weight, and patient response.
Initial dosing in a naïve patient should begin at the lowest effective dose.
To minimise the incidence of dysphagia, the sternomastoid should not be injected bilaterally.
The following doses are recommended:
| Type I Head rotated toward side of shoulder elevation | Sternomastoid | 50-100 Units; at least 2 sites |
| Levator scapulae | 50 Units; 1-2 sites | |
| Scalene | 25-50 Units; 1-2 sites | |
| Splenius capitis | 25-75 Units; 1-3 sites | |
| Trapezius | 25-100 Units; 1-8 sites | |
| Type II Head rotation only | Sternomastoid | 25-100 Units; at least 2 sites if >25 Units given |
| Type III Head tilted toward side of shoulder elevation | Sternomastoid | 25-100 Units at posterior border; at least 2 sites if >25 Units given |
| Levator scapulae | 25-100 Units; at least 2 sites | |
| Scalene | 25-75 Units; at least 2 sites | |
| Trapezius | 25-100 Units; 1 – 8 sites | |
| Type IV Bilateral posterior cervical muscle spasm with elevation of the face | Splenius capitis and cervicis | 50-200 Units; 2-8 sites, treat bilaterally(This is the total dose and not the dose for each side of the neck) |
Maximum dose:
No more than 50 Units should be given at any one injection site.
No more than 100 Units should be given to the sternomastoid.
No more than 200 Units in total should be injected for the first course of therapy, with adjustments made in subsequent courses dependent on the initial response, up to a maximum total dose of 300 Units.
Additional information: Treatment intervals of less than 10 weeks are not recommended.
Chronic migraine
Recommended needle: Sterile 30 gauge, 0.5 inch needle.
A 1 inch needle may be needed in the neck region for patients with extremely thick neck muscles.
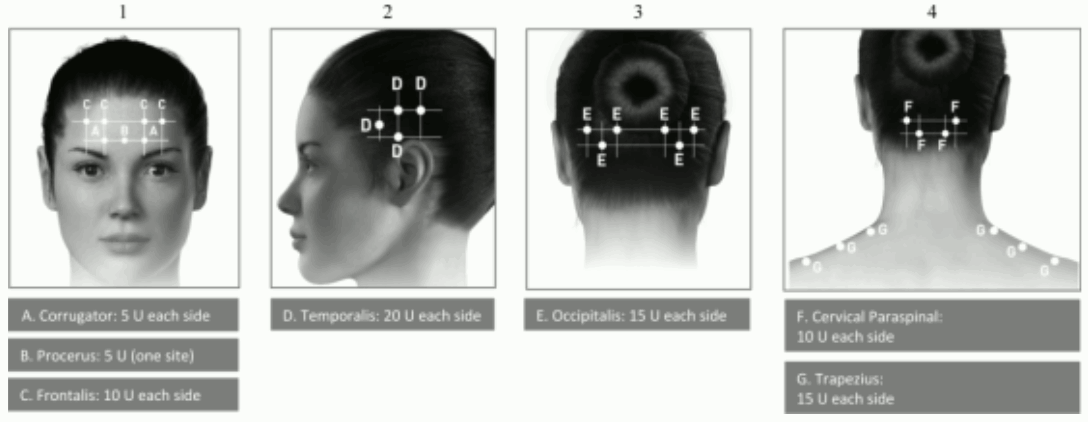
Administration guidance: Injections should be divided across 7 specific head/neck muscle areas as specified in the diagrams below. With the exception of the procerus muscle, which should be injected at 1 site (midline), all muscles should be injected bilaterally with half the number of injection sites administered to the left, and half to the right side of the head and neck.
The following diagrams indicate the injection sites:
If there is a predominant pain location(s), additional injections to one or both sides may be administered in up to 3 specific muscle groups (occipitalis, temporalis and trapezius), up to the maximum dose per muscle as indicated in the table below.
The following diagrams indicate recommended muscle groups for optional additional injections:
Recommended dose: 155 Units to 195 Units administered intramuscularly as 0.1 ml (5 Units) injections to 31 and up to 39 sites.
| Recommended Dose | |
|---|---|
| Head/Neck Area | Total Dosage (number of sites*) |
| Corrugator** | 10 Units (2 sites) |
| Procerus | 5 Units (1 site) |
| Frontalis** | 20 Units (4 sites) |
| Temporalis** | 40 Units (8 sites) up to 50 Units (up to 10 sites) |
| Occipitalis** | 30 Units (6 sites) up to 40 Units (up to 8 sites) |
| Cervical Paraspinal Muscle Group** | 20 Units (4 sites) |
| Trapezius** | 30 Units (6 sites) up to 50 Units (up to 10 sites) |
| Total Dose Range: | 155 Units to 195 Units 31 to 39 sites |
* 1 IM injection site = 0.1 ml = 5 Units BOTOX
** Dose distributed bilaterally
Additional information: The recommended re-treatment schedule is every 12 weeks.
BLADDER DISORDERS
Overactive bladder
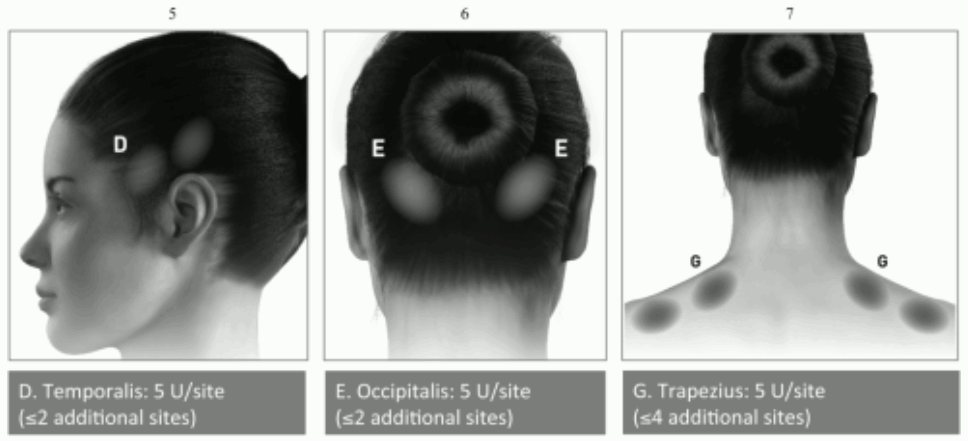
Recommended needle: The injection needle should be filled (primed) with approximately 1 ml of the reconstituted BOTOX solution prior to the start of the injections (depending on the needle length) to remove any air.
Administration guidance: The reconstituted solution of BOTOX (100 Units/10 ml) is injected via a flexible or rigid cystoscope, avoiding the trigone and base. The bladder should be instilled with enough saline to achieve adequate visualisation for the injections and avoid backflow of the product, but over-distension should be avoided.
The needle should be inserted approximately 2 mm into the detrusor, and 20 injections of 0.5 ml each (total volume 10 ml) should be spaced approximately 1 cm apart (see figure below). For the final injection, approximately 1 ml of sterile unpreserved normal saline (0.9% sodium chloride for injection) should be injected so the full dose is delivered.
Recommended dose: The recommended dose is 100 Units of BOTOX, as 0.5 ml (5 Units) injections across 20 sites in the detrusor muscle.
Additional information: For the patient preparation and monitoring, see section 4.4.
After the injections are given, the saline used for bladder wall visualisation should not be drained so that the patients can demonstrate their ability to void prior to leaving the clinic. The patient should be observed for at least 30 minutes post-injection and until a spontaneous void has occurred.
Patients should be considered for reinjection when the clinical effect of the previous injection has diminished but no sooner than 3 months from the prior bladder injection.
Urinary incontinence due to neurogenic detrusor overactivity
Recommended needle: The injection needle should be filled (primed) with approximately 1 ml of the reconstituted BOTOX solution prior to the start of the injections (depending on the needle length) to remove any air.
Administration guidance: The reconstituted solution of BOTOX (200 Units/30 ml) is injected via a flexible or rigid cystoscope, avoiding the trigone and base. The bladder should be instilled with enough saline to achieve adequate visualisation for the injections and avoid backflow of the product, but over-distension should be avoided.
The needle should be inserted approximately 2 mm into the detrusor, and 30 injections of 1 ml each (total volume 30 ml) should be spaced approximately 1 cm apart (see figure above). For the final injection, approximately 1 ml of sterile unpreserved normal saline (0.9% sodium chloride for injection) should be injected so the full dose is delivered. After the injections are given, the saline used for bladder wall visualisation should be drained.
Recommended dose: The recommended dose is 200 Units of BOTOX, as 1 ml (~6.7 Units) injections across 30 sites in the detrusor muscle.
Additional information: For the patient preparation and monitoring, see section 4.4.
Patients should be considered for reinjection when the clinical effect of the previous injection has diminished, but no sooner than 3 months from the prior bladder injection.
No urodynamic data beyond 2 treatments and no histopathological data after repeated treatment are currently available.
Patients should not receive multiple treatments in the event of limited symptomatic improvement.
SKIN AND SKIN APPENDAGE DISORDERS
Primary hyperhidrosis of the axillae
Recommended needle: Sterile 30 gauge needle.
Administration guidance: The hyperhidrotic area to be injected may be defined by using standard staining techniques, e.g. Minor´s iodine-starch test.
Recommended dose: 50 Units of BOTOX is injected intradermally to each axilla, evenly distributed in multiple sites approximately 1-2 cm apart.
The recommended injection volume for intradermal injection is 0.1-0.2 ml.
Maximum dose: Doses other than 50 Units per axilla cannot be recommended.
Additional information: Clinical improvement generally occurs within the first week after injection and persists for 4-7 months.
Repeat injection of BOTOX can be administered when the clinical effect of a previous injection diminishes and the treating physician deems it necessary. Injections should not be repeated more frequently than every 16 weeks.
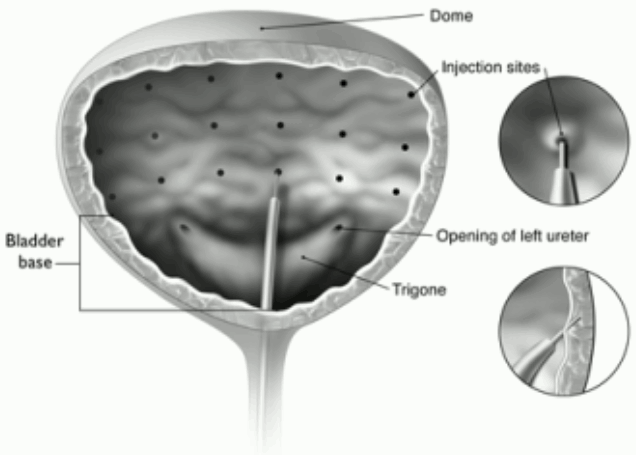
Glabellar lines seen at maximum frown
Recommended needle: Sterile 30 gauge needle.
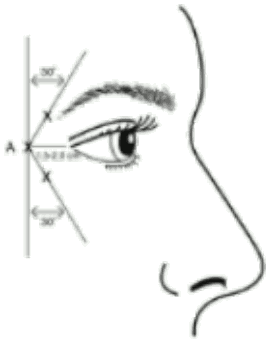
Administration guidance: Before injection, the thumb or index finger is to be placed firmly below the orbital rim in order to prevent extravasation below the orbital rim. The needle should be oriented superiorly and medially during the injection. In addition, injections near the levator palpebrae superioris muscle must be avoided, particularly in patients with larger brow-depressor complexes (depressor supercilii). Injections in the corrugator muscle must be done in the central part of that muscle, a distance of at least 1 cm above the arch of the eyebrows (see figure).
Care should be taken to ensure that BOTOX is not injected into a blood vessel when it is injected in the glabellar lines seen at maximum frown, see section 4.4.
Recommended dose: A volume of 0.1 ml (4 Units) is administered in each of the 5 injection sites (see Figure): 2 injections in each corrugator muscle and 1 injection in the procerus muscle for a total dose of 20 Units.
Maximum dose: In order to reduce the risk of eyelid ptosis, the maximum dose of 4 Units for each injection site as well as the number of injection sites should not be exceeded.
Additional information:
Treatment intervals should not be more frequent than every three months. In the event of treatment failure or diminished effect following repeat injections, alternative treatment methods should be employed.
In case of insufficient dose a second treatment session should be initiated by adjusting the total dose up to 40 or 50 Units, taking into account the analysis of the previous treatment failure (see information in All indications).
The efficacy and safety of repeat injections of BOTOX for the treatment of glabellar lines beyond 12 months has not been evaluated.
Crow’s feet lines seen at maximum smile
Recommended needle: Sterile 30 gauge needle.
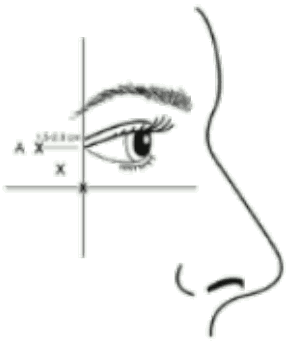
Administration guidance: Injections should be given with the needle tip bevel up and oriented away from the eye. The first injection (A) should be made approximately 1.5 to 2.0 cm temporal to the lateral canthus and just temporal to the orbital rim. If the lines in the crow’s feet region are above and below the lateral canthus, inject as shown in Figure 1. Alternatively, if the lines in the crow’s feet region are primarily below the lateral canthus, inject as shown in Figure 2.
In order to reduce the risk of eyelid ptosis, injections should be made temporal to the orbital rim, thereby maintaining a safe distance from the muscle controlling eyelid elevation.
Figure 1:
Figure 2:
Care should be taken to ensure that BOTOX is not injected into a blood vessel when it is injected in the crow’s feet lines seen at maximum smile (see section 4.4).
Recommended dose: A volume of 0.1 ml (4 Units) is administered in each of the 3 injection sites per side (total of 6 injection sites) in the lateral orbicularis oculi muscle, for a total dose of 24 Units in a total volume of 0.6 ml (12 Units per side).
For simultaneous treatment with glabellar lines seen at maximum frown, the dose is 24 Units for crow’s feet lines seen at maximum smile and 20 Units for glabellar lines (see Administration guidance for glabellar lines) for a total dose of 44 Units in a total volume of 1.1 ml.
Maximum dose: In order to reduce the risk of eyelid ptosis, the maximum dose of 4 Units for each injection site as well as the number of injection sites should not be exceeded.
Additional information: Treatment intervals should not be more frequent than every 3 months.
The efficacy and safety of repeat injections of BOTOX for the treatment of crow’s feet lines beyond 12 months has not been evaluated.
Forehead Lines seen at maximum eyebrow elevation
Recommended needle: Sterile 30 gauge needle.
Administration guidance:
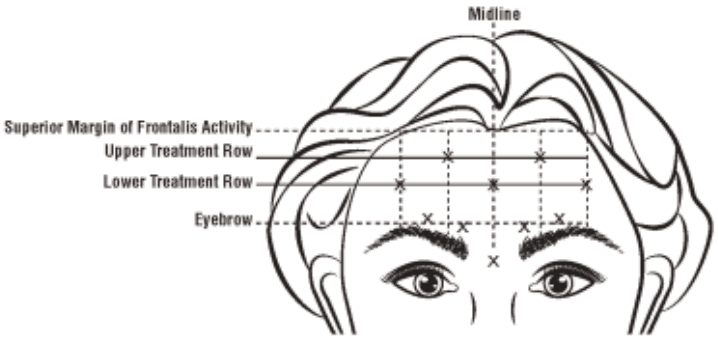
To identify the location of the appropriate injection sites in the frontalis muscle, assess the overall relationship between the size of the subject’s forehead, and the distribution of frontalis muscle activity should be assessed.
The following horizontal treatment rows should be located by light palpation of the forehead at rest and maximum eyebrow elevation:
- Superior Margin of Frontalis Activity: approximately 1 cm above the most superior forehead crease
- Lower Treatment Row: midway between the superior margin of frontalis activity and the eyebrow, at least 2 cm above the eyebrow
- Upper Treatment Row: midway between the superior margin of frontalis activity and lower treatment row
The 5 injections should be placed at the intersection of the horizontal treatment rows with the following vertical landmarks:
- On the lower treatment row at the midline of the face, and 0.5-1.5 cm medial to the palpated temporal fusion line (temporal crest); repeat for the other side.
- On the upper treatment row, midway between the lateral and medial sites on the lower treatment row; repeat for the other side.
Figure 3:
Care should be taken to ensure that BOTOX is not injected into a blood vessel when it is injected in the forehead lines seen at maximum eyebrow elevation (see section 4.4).
Recommended dose:
A volume of 0.1 ml (4 Units) is administered in each of the 5 injection sites in the frontalis muscle, for a total dose of 20 Units in a total volume of 0.5 ml (see Figure 3).
The total dose for treatment of forehead lines (20 Units) in conjunction with glabellar lines (20 Units) is 40 Units/1.0 mL.
For simultaneous treatment with glabellar lines and crow’s feet lines, the total dose is 64 Units, comprised of 20 Units for forehead lines, 20 Units for glabellar lines (see Recommended dose for Glabellar Lines and Figure), and 24 Units for crow’s feet lines (see Recommended dose for Crow’s Feet Lines and Figures 1 and 2).
Additional information: Treatment intervals should not be more frequent than every 3 months.
The efficacy and safety of repeat injections of BOTOX for the treatment of forehead lines beyond 12 months has not been evaluated.
ALL INDICATIONS
In case of treatment failure after the first treatment session, i.e. absence, at one month after injection, of significant clinical improvement from baseline, the following actions should be taken:
- Clinical verification, which may include electromyographic examination in a specialist setting, of the action of the toxin on the injected muscle(s);
- Analysis of the causes of failure, e.g. bad selection of muscles to be injected, insufficient dose, poor injection technique, appearance of fixed contracture, antagonist muscles too weak, formation of toxin-neutralising antibodies;
- Re-evaluation of the appropriateness of treatment with botulinum toxin type A;
- In the absence of any undesirable effects secondary to the first treatment session, instigate a second treatment session as following: i) adjust the dose, taking into account the analysis of the earlier treatment failure; ii) use EMG; and iii) maintain a three-month interval between the two treatment sessions.
In the event of treatment failure or diminished effect following repeat injections alternative treatment methods should be employed.
When treating adult patients for multiple indications, the maximum cumulative dose should not exceed 400 Units in a 12-week interval.
In treating paediatric patients, including when treating for multiple indications, the maximum cumulative dose should not exceed the lower of 10 Units/kg body weight or 340 Units, in a 12-week interval.
Overdose
Overdose of BOTOX is a relative term and depends upon dose, site of injection, and underlying tissue properties. No cases of systemic toxicity resulting from accidental injection of BOTOX have been observed. Excessive doses may produce local, or distant, generalised and profound neuromuscular paralysis. No cases of ingestion of BOTOX have been reported.
Signs and symptoms of overdose are not apparent immediately post-injection. Should accidental injection or ingestion occur or overdose be suspected, the patient should be medically monitored for up to several weeks for progressive signs and symptoms of muscular weakness, which could be local or distant from the site of injection and may include ptosis, diplopia, dysphagia, dysarthria, generalised weakness or respiratory failure. These patients should be considered for further medical evaluation and appropriate medical therapy immediately instituted, which may include hospitalisation.
If the musculature of the oropharynx and oesophagus are affected, aspiration may occur which may lead to development of aspiration pneumonia. If the respiratory muscles become paralysed or sufficiently weakened, intubation and assisted respiration will be required until recovery takes place and may involve the need for a tracheostomy and prolonged mechanical ventilation, in addition to other general supportive care.
Shelf life
3 years.
After reconstitution, stability has been demonstrated for 24 hours at 2°C–8°C.
From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2°C to 8°C (see also section 6.6).
Special precautions for storage
Store in a refrigerator (2°C-8°C), or store in a freezer (-5°C to -20°C).
For storage conditions of the reconstituted medicinal product see section 6.3.
Nature and contents of container
Clear glass vial, with rubber stopper and tamper-proof aluminium seal, containing white powder for solution for injection.
Pack size:
- Carton comprising one 100 Allergan Unit vial and package leaflet.
- Packs containing one, two, three or six cartons.
Not all pack sizes may be marketed.
Special precautions for disposal and other handling
Reconstitution
BOTOX is reconstituted prior to use with sterile unpreserved normal saline (0.9% sodium chloride for injection). It is good practice to perform vial reconstitution and syringe preparation over plastic-lined paper towels to catch any spillage. An appropriate amount of diluent (see dilution table below) is drawn up into a syringe. The exposed portion of the rubber septum of the vial is cleaned with alcohol (70%) prior to insertion of the needle. Since BOTOX is denatured by bubbling or similar violent agitation, the diluent should be injected gently into the vial. Discard the vial if a vacuum does not pull the diluent into the vial. Reconstituted BOTOX is a clear colourless to slightly yellow solution free of particulate matter. When reconstituted, BOTOX may be stored in a refrigerator (2-8°C) for up to 24 hours prior to use. After this period used or unused vials should be discarded.
Each vial is for single use only.
Care should be taken to use the correct diluent volume for the presentation chosen to prevent accidental overdose. If different vial sizes of BOTOX are being used as part of one injection procedure, care should be taken to use the correct amount of diluent when reconstituting a particular number of units per 0.1 ml. The amount of diluent varies between BOTOX 50 Allergan Units, BOTOX 100 Allergan Units and BOTOX 200 Allergan Units. Each syringe should be labelled accordingly.
Dilution table for BOTOX 50, 100 and 200 Allergan Units vial size for all indications except bladder disorders:
| 50 Unit vial | 100 Unit vial | 200 Unit vial | |
|---|---|---|---|
| Resulting dose (Units per 0.1 ml) | Amount of diluent (sterile unpreserved normal saline (0.9% sodium chloride for injection)) added in a 50 Unit vial | Amount of diluent (sterile unpreserved normal saline (0.9% sodium chloride for injection)) added in a 100 Unit vial | Amount of diluent (sterile unpreserved normal saline (0.9% sodium chloride for injection)) added in a 200 Unit vial |
| 20 Units | 0.25 ml | 0.5 ml | 1 ml |
| 10 Units | 0.5 ml | 1 ml | 2 ml |
| 5 Units | 1 ml | 2 ml | 4 ml |
| 4 Units | 1.25 ml | 2.5 ml | 5 ml |
| 2.5 Units | 2 ml | 4 ml | 8 ml |
| 1.25 Units | 4 ml | 8 ml | N/A |
Overactive bladder
It is recommended that a 100 Unit or two 50 Unit vials are used for convenience of reconstitution.
Dilution instructions using two 50 Unit vials:
- Reconstitute two 50 Unit vials of BOTOX each with 5 ml of sterile unpreserved normal saline (0.9% sodium chloride for injection) and mix the vials gently.
- Draw the 5 ml from each of the vials into a single 10 ml syringe.
This will result in a 10 ml syringe containing a total of 100 Units of reconstituted BOTOX. Use immediately after reconstitution in the syringe. Dispose of any unused saline.
Dilution instructions using a 100 Unit vial:
- Reconstitute a 100 Unit vial of BOTOX with 10 ml of sterile unpreserved normal saline (0.9% sodium chloride for injection) and mix gently.
- Draw the 10 ml from the vial into a 10 ml syringe.
This will result in a 10 ml syringe containing a total of 100 Units of reconstituted BOTOX. Use immediately after reconstitution in the syringe. Dispose of any unused saline.
Dilution instructions using a 200 Unit vial:
- Reconstitute a 200 Unit vial of BOTOX with 8 ml of sterile unpreserved normal saline (0.9% sodium chloride for injection) and mix gently.
- Draw 4 ml from the vial into a 10 ml syringe.
- Complete the reconstitution by adding 6 ml of sterile unpreserved normal saline (0.9% sodium chloride for injection) into the 10 ml syringe and mix gently.
This will result in a 10 ml syringe containing a total of 100 Units of reconstituted BOTOX. Use immediately after reconstitution in the syringe. Dispose of any unused saline.
This product is for single use only and any unused reconstituted product should be disposed of.
Urinary incontinence due to neurogenic detrusor overactivity
It is recommended that a 200 Unit vial or two 100 Unit vials are used for convenience of reconstitution.
Dilution instructions using four 50 Unit vials:
- Reconstitute four 50 Unit vials of BOTOX, each with 3 ml of sterile unpreserved normal saline (0.9% sodium chloride for injection) and mix the vials gently.
- Draw 3 ml from the first vial and 1 ml from the second vial into one 10 ml syringe.
- Draw 3 ml from the third vial and 1 ml from the fourth vial into a second 10 ml syringe.
- Draw the remaining 2 ml from the second and fourth vials into a third 10 ml syringe.
- Complete the reconstitution by adding 6 ml of sterile unpreserved normal saline (0.9% sodium chloride for injection) into each of the three 10 ml syringes, and mix gently.
This will result in three 10 ml syringes containing a total of 200 Units of reconstituted BOTOX. Use immediately after reconstitution in the syringe. Dispose of any unused saline.
Dilution instructions using two 100 Unit vials:
- Reconstitute two 100 Unit vials of BOTOX, each with 6 ml of sterile unpreserved normal saline (0.9% sodium chloride for injection) and mix the vials gently.
- Draw 4 ml from each vial into each of two 10 ml syringes.
- Draw the remaining 2 ml from each vial into a third 10 ml syringe.
- Complete the reconstitution by adding 6 ml of sterile unpreserved normal saline (0.9% sodium chloride for injection) into each of the 10 ml syringes, and mix gently.
This will result in three 10 ml syringes containing a total of 200 Units of reconstituted BOTOX. Use immediately after reconstitution in the syringe. Dispose of any unused saline.
Dilution instructions using a 200 Unit vial:
- Reconstitute a 200 Unit vial of BOTOX with 6 ml of sterile unpreserved normal saline (0.9% sodium chloride for injection) and mix the vials gently.
- Draw 2 ml from the vial into each of three 10 ml syringes.
- Complete the reconstitution by adding 8 ml of sterile unpreserved normal saline (0.9% sodium chloride for injection) into each of the 10 ml syringes, and mix gently.
This will result in three 10 ml syringes containing a total of 200 Units of reconstituted BOTOX. Use immediately after reconstitution in the syringe. Dispose of any unused saline.
The ‘unit’ by which the potency of preparations of BOTOX is measured should be used to calculate dosages of BOTOX only and is not transferable to other preparations of botulinum toxin.
Disposal
For safe disposal, unused vials should be reconstituted with a small amount of water then autoclaved. Any used vials, syringes, and spillages etc. should be autoclaved, or the residual BOTOX inactivated using dilute hypochlorite solution (0.5%).
Any unused product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.