BREVINOR Tablet Ref.[27868] Active ingredients: 17 alpha-Ethinylestradiol Norethisterone
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2019 Publisher: Pfizer Limited, Ramsgate Road, Sandwich, Kent CT13 9NJ, UK
4.3. Contraindications
Hypersensitivity to the active substances or to any of the excipients listed in section 6.1.
As with all combined progestogen/oestrogen oral contraceptives, the following conditions should be regarded as contra-indications:
- History of confirmed venous thromboembolic disease (VTE), family history of idiopathic VTE and other known risk factors of VTE
- Thrombophlebitis, cerebrovascular disorders, coronary artery disease, myocardial infarction, angina, hyperlipidaemia or a history of these conditions.
- Acute or severe chronic liver disease, including liver tumours, Dubin-Johnson or Rotor syndrome.
- History during pregnancy of idiopathic jaundice, severe pruritus or pemphigoid gestationis.
- Known or suspected breast or genital cancer.
- Known or suspected oestrogen-dependent neoplasia.
- Undiagnosed abnormal vaginal bleeding.
- A history of migraines classified as classical focal or crescendo.
- Pregnancy.
Brevinor is contraindicated for concomitant use with the medicinal products containing ombitasvir/paritaprevir/ritonavir and/or dasabuvir, (see sections 4.4 and section 4.5).
4.4. Special warnings and precautions for use
Assessment of women prior to starting oral contraceptives (and at regular intervals thereafter) should include a personal and family medical history of each woman. Physical examination should be guided by this and by the contraindications (section 4.3) and warnings (section 4.4) for this product. The frequency and nature of these assessments should be based upon relevant guidelines and should be adapted to the individual woman, but should include measurement of blood pressure and, if judged appropriate by the clinician, breast, abdominal and pelvic examination including cervical cytology.
Women taking oral contraceptives require careful observation if they have or have had any of the following conditions: breast nodules; fibrocystic disease of the breast or an abnormal mammogram; uterine fibroids; a history of severe depressive states; varicose veins; sickle-cell anaemia; diabetes; hypertension; cardiovascular disease; migraine; epilepsy; asthma; otosclerosis; multiple sclerosis; porphyria; tetany; disturbed liver functions; gallstones; kidney disease; chloasma; any condition that is likely to worsen during pregnancy. The worsening or first appearance of any of these conditions may indicate that the oral contraceptive should be stopped. Discontinue treatment if there is a gradual or sudden, partial or complete loss of vision or any evidence of ocular changes, onset or aggravation of migraine or development of headache of a new kind, which is recurrent, persistent or severe.
Gastro-intestinal upsets, such as vomiting and diarrhoea, may interfere with the absorption of the tablets leading to a reduction in contraceptive efficacy. Patients should continue to take Brevinor, but they should also be encouraged to use another contraceptive method during the period of gastro-intestinal upset and for the next 7 days.
Progestogen oestrogen preparations should be used with caution in patients with a history of hepatic dysfunction or hypertension.
An increased risk of venous thromboembolic disease (VTE) associated with the use of oral contraceptives is well established but is smaller than that associated with pregnancy, which has been estimated at 60 cases per 100,000 pregnancies. Some epidemiological studies have reported a greater risk of VTE for women using combined oral contraceptives containing desogestrel or gestodene (the so-called 'third generation' pills) than for women using pills containing levonorgestrel or norethisterone (the so-called 'second generation' pills
The spontaneous incidence of VTE in healthy non-pregnant women (not taking any oral contraceptive) is about 5 cases per 100,000 per year. The incidence in users of second generation pills is about 15 per 100,000 women per year of use. The incidence in users of third generation pills is about 25 cases per 100,000 women per year of use; this excess incidence has not been satisfactorily explained by bias or confounding. The level of all of these risks of VTE increases with age and is likely to be further increased in women with other known risk factors for VTE such as obesity. The excess risk of VTE is highest during the first year a woman ever uses a combined oral contraceptive.
Patients receiving oral contraceptives should be kept under regular surveillance, in view of the possibility of development of conditions such as thromboembolism.
The risk of coronary artery disease in women taking oral contraceptives is increased by the presence of other predisposing factors such as cigarette smoking, hypercholesterolemia, obesity, diabetes, history of pre-eclamptic toxaemia and increasing age. After the age of thirty-five years, the patient and physician should carefully re-assess the risk/benefit ratio of using combined oral contraceptives as opposed to alternative methods of contraception.
Brevinor should be discontinued at least four weeks before, and for two weeks following, elective operations and during immobilisation. Patients undergoing injection treatment for varicose veins should not resume taking Brevinor until 3 months after the last injection.
Benign and malignant liver tumours have been associated with oral contraceptive use. The relationship between occurrence of liver tumours and use of female sex hormones is not known at present. These tumours may rupture causing intra-abdominal bleeding. If the patient presents with a mass or tenderness in the right upper quadrant or an acute abdomen, the possible presence of a tumour should be considered.
The risk of arterial thrombosis associated with combined oral contraceptives increases with age, and this risk is aggravated by cigarette smoking. The use of combined oral contraceptives by women in the older age group, especially those who are cigarette smokers, should therefore be discouraged and alternative methods advised.
The use of this product in patients suffering from epilepsy, migraine, asthma or cardiac dysfunction may result in exacerbation of these disorders because of fluid retention. Caution should also be observed in patients who wear contact lenses.
Decreased glucose tolerance may occur in diabetic patients on this treatment, and their control must be carefully supervised.
The use of oral contraceptives has also been associated with a possible increased incidence of gall bladder disease.
Women with a history of oligomenorrhoea or secondary amenorrhoea or young women without regular cycles may have a tendency to remain anovulatory or to become amenorrhoeic after discontinuation of oral contraceptives. Women with these pre-existing problems should be advised of this possibility and encouraged to use other contraceptive methods.
Numerous epidemiological studies have been reported on the risks of ovarian, endometrial, cervical and breast cancer in women using combined oral contraceptives. The evidence is clear that combined oral contraceptives offer substantial protection against both ovarian and endometrial cancer.
An increased risk of cervical cancer in long-term users of combined oral contraceptives has been reported in some studies, but there continues to be controversy about the extent to which this is attributable to the confounding effects of sexual behaviour and other factors.
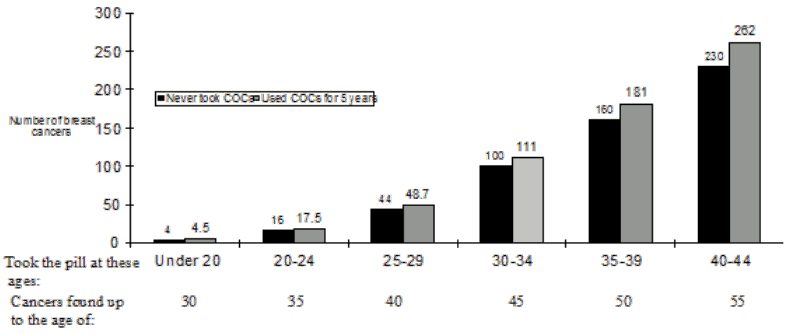
A meta-analysis from 54 epidemiological studies reported that there is a slightly increased relative risk (RR = 1.24) of having breast cancer diagnosed in women who are currently using combined oral contraceptives (COCs). The observed pattern of increased risk may be due to an earlier diagnosis of breast cancer in COC users, the biological effects of COCs or a combination of both. The additional breast cancers diagnosed in current users of COCs or in women who have used COCs in the last ten years are more likely to be localised to the breast than those in women who never used COCs.
Breast cancer is rare among women under 40 years of age whether or not they take COCs. Whilst this background risk increases with age, the excess number of breast cancer diagnoses in current and recent COC users is small in relation to the overall risk of breast cancer (see bar chart).
The most important risk factor for breast cancer in COC users is the age women discontinue the COC; the older the age at stopping, the more breast cancers are diagnosed. Duration of use is less important and the excess risk gradually disappears during the course of the 10 years after stopping COC use such that by 10 years there appears to be no excess.
The possible increase in risk of breast cancer should be discussed with the user and weighed against the benefits of COCs taking into account the evidence that they offer substantial protection against the risk of developing certain other cancers (e.g. ovarian and endometrial cancer).
Estimated cumulative numbers of breast cancers per 10,000 women diagnosed in 5 years of use and up to 10 years after stopping COCs, compared with numbers of breast cancers diagnosed in 10,000 women who had never used COCs.
ALT elevations
During clinical trials with patients treated for hepatitis C virus infections (HCV) with the medicinal products containing ombitasvir/paritaprevir/ritonavir and dasabuvir with or without ribavirin, transaminase (ALT) elevations higher than 5 times the upper limit of normal (ULN) occurred significantly more frequent in women using ethinylestradiol-containing medications such as combined hormonal contraceptives (CHCs) (see sections 4.3 and 4.5).
Depressed mood and depression are well-known undesirable effects of hormonal contraceptive use (see section 4.8). Depression can be serious and is a well-known risk factor for suicidal behaviour and suicide. Women should be advised to contact their physician in case of mood changes and depressive symptoms, including shortly after initiating the treatment.
4.5. Interaction with other medicinal products and other forms of interaction
The herbal remedy St John's wort (Hypericum perforatum) should not be taken concomitantly with this medicine as this could potentially lead to a loss of contraceptive effect.
Some drugs may modify the metabolism of Brevinor reducing its effectiveness; these include certain sedatives, antibiotics, anti-epileptic and anti-arthritic drugs. During the time such agents are used concurrently, it is advised that mechanical contraceptives also be used.
The results of a large number of laboratory tests have been shown to be influenced by the use of oestrogen containing oral contraceptives, which may limit their diagnostic value. Among these are: biochemical markers of thyroid and liver function; plasma levels of carrier proteins, triglycerides, coagulation and fibrinolysis factors.
Pharmacodynamic interactions
Concomitant use with the medicinal products containing ombitasvir/paritaprevir/ritonavir and dasabuvir, with or without ribavirin may increase the risk of ALT elevations (see sections 4.3 and 4.4). Therefore, Brevinor-users must switch to an alternative method of contraception (e.g., progestogen-only contraception or non-hormonal methods) prior to starting therapy with this combination drug regimen. Brevinor can be restarted 2 weeks following completion of treatment with this combination drug regimen.
4.6. Pregnancy and lactation
Pregnancy
Brevinor is not indicated during pregnancy. If pregnancy occurs during medication with Brevinor, treatment should be withdrawn immediately. Like all norethisterone derivatives used for contraception, Brevinor has slight androgenic activity. At doses higher than normally used in OC and HRT formulations, masculinisation of female foetuses has been observed. The results of most epidemiological studies to date relevant to inadvertent foetal exposure to combinations of oestrogens with progestogens, indicate no teratogenic or foetotoxic effects.
Breast-feeding
Patients who are fully breast-feeding should not take Brevinor tablets since, in common with other combined oral contraceptives, the oestrogen component may reduce the amount of milk produced. In addition, active ingredients or their metabolites have been detected in the milk of mothers taking oral contraceptives. The effect of Brevinor on breast-fed infants has not been determined.
4.7. Effects on ability to drive and use machines
Not relevant.
4.8. Undesirable effects
As with all oral contraceptives, there may be slight nausea at first, weight gain or breast discomfort, which soon disappear.
Other side-effects known or suspected to occur with oral contraceptives include gastro-intestinal symptoms, changes in libido and appetite, headache, exacerbation of existing uterine fibroid disease, depression, and changes in carbohydrate, lipid and vitamin metabolism.
Spotting or bleeding may occur during the first few cycles. Usually menstrual bleeding becomes light and occasionally there may be no bleeding during the tablet-free days.
Hypertension, which is usually reversible on discontinuing treatment, has occurred in a small percentage of women taking oral contraceptives.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store.
6.2. Incompatibilities
Not applicable.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.