BREYANZI Dispersion for infusion Ref.[49783] Active ingredients: Lisocabtagene maraleucel
Source: European Medicines Agency (EU) Revision Year: 2025 Publisher: Bristol-Myers Squibb Pharma EEIG, Plaza 254, Blanchardstown Corporate Park 2, Dublin 15, D15 T867, Ireland
4.1. Therapeutic indications
Breyanzi is indicated for the treatment of adult patients with diffuse large B-cell lymphoma (DLBCL), high grade B-cell lymphoma (HGBCL), primary mediastinal large B-cell lymphoma (PMBCL) and follicular lymphoma grade 3B (FL3B), who relapsed within 12 months from completion of, or are refractory to, first-line chemoimmunotherapy.
Breyanzi is indicated for the treatment of adult patients with relapsed or refractory DLBCL, PMBCL and FL3B, after two or more lines of systemic therapy.
Breyanzi is indicated for the treatment of adult patients with relapsed or refractory follicular lymphoma (FL) after two or more lines of systemic therapy.
Breyanzi is indicated for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (MCL) after at least two lines of systemic therapy including a Bruton's tyrosine kinase (BTK) inhibitor.
4.2. Posology and method of administration
Breyanzi must be administered in a qualified treatment centre.
Treatment should be initiated under the direction of and supervised by a healthcare professional experienced in the treatment of haematological malignancies and trained for administration and management of patients treated with Breyanzi.
At least 1 dose of tocilizumab for use in the event of cytokine release syndrome (CRS) and emergency equipment must be available per patient prior to infusion of Breyanzi. The treatment centre must have access to an additional dose of tocilizumab within 8 hours of each previous dose. In the exceptional case where tocilizumab is not available due to a shortage that is listed in the European Medicines Agency shortage catalogue, suitable alternative measures to treat CRS instead of tocilizumab must be available prior to infusion.
Posology
Breyanzi is intended for autologous use (see section 4.4).
Treatment consists of a single dose for infusion containing a dispersion for infusion of CAR-positive viable T-cells in one or more vials.
The target dose is 100 × 106 CAR-positive viable T-cells (consisting of a target 1:1 ratio of CD4+ and CD8+ cell components) within a range of 44-120 × 106 CAR-positive viable T-cells. See the accompanying release for infusion certificate (RfIC) for additional information pertaining to dose.
The availability of Breyanzi must be confirmed before starting lymphodepleting chemotherapy regimen.
Patients should be clinically re-assessed prior to administration of lymphodepleting chemotherapy and Breyanzi to ensure no reasons to delay therapy (see section 4.4).
Pre-treatment (lymphodepleting chemotherapy)
Lymphodepleting chemotherapy consisting of cyclophosphamide 300 mg/m²/day and fludarabine 30 mg/m²/day, administered intravenously for three days. See the prescribing information for fludarabine and cyclophosphamide for information on dose adjustment in renal impairment.
Breyanzi is to be administered 2 to 7 days after completion of lymphodepleting chemotherapy.
If there is a delay of more than 2 weeks between completing lymphodepleting chemotherapy and the infusion of Breyanzi, then the patient should be re-treated with lymphodepleting chemotherapy prior to receiving the infusion (see section 4.4).
Pre-medication
It is recommended that premedication with paracetamol and diphenhydramine (25-50 mg, intravenously or orally) or another H1-antihistamine, be administered 30 to 60 minutes before the infusion of Breyanzi to reduce the possibility of an infusion reaction.
Prophylactic use of systemic corticosteroids should be avoided, as the use may interfere with the activity of Breyanzi (see section 4.4).
Monitoring after infusion
- Patients should be monitored 2-3 times during the first week following infusion, for signs and symptoms of potential CRS, neurologic events and other toxicities. Physicians should consider hospitalisation at the first signs or symptoms of CRS and/or neurologic events.
- Frequency of monitoring after the first week should be carried out at the physician's discretion and should be continued for at least 2 weeks after infusion.
- Patients should be instructed to remain within proximity of a qualified treatment centre for at least 2 weeks following infusion.
Special populations
Patients with human immunodeficiency virus (HIV), hepatitis B virus (HBV) and hepatitis C virus (HCV) infection
There is no clinical experience in patients with active HIV, HBV or HCV infection.
Screening for HIV, active HBV and active HCV must be performed before collection of cells for manufacturing. Leukapheresis material from patients with active HIV or active HCV infection will not be accepted for manufacturing (see section 4.4).
Renal impairment
There is no clinical experience in patients with severe renal impairment (creatinine clearance ≤30 mL/min).
Elderly
No dose adjustment is required in patients over 65 years of age.
Paediatric population
The safety and efficacy of Breyanzi in children and adolescents below 18 years of age have not yet been established. No data are available.
Method of administration
Breyanzi is for intravenous use only.
Preparation of Breyanzi
Before thawing the vials, it must be confirmed that the patient's identity matches the unique patient identifiers on the shipper, external carton and the release for infusion certificate (RfIC). The total number of vials to be administered must also be confirmed with the patient-specific label information on the release for infusion certificate (RfIC) (see section 4.4). The company must be contacted immediately if there are any discrepancies between the labels and the patient identifiers.
Administration
- Do NOT use a leukodepleting filter.
- Ensure tocilizumab or suitable alternatives, in the exceptional case where tocilizumab is not available due to a shortage that is listed in the European Medicines Agency shortage catalogue, and emergency equipment are available prior to infusion and during the recovery period.
- Confirm the patient's identity matches the patient identifiers on the syringe label supplied on the respective release for infusion certificate (RfIC).
- Once Breyanzi components have been drawn into syringes, proceed with administration as soon as possible. The total time from removal from frozen storage to patient administration should not exceed 2 hours.
For detailed instructions on preparation, administration, measures to take in case of accidental exposure and disposal of Breyanzi, see section 6.6.
4.9. Overdose
No data from clinical studies are available regarding overdose of Breyanzi.
6.3. Shelf life
Unopened vial when stored in the vapour phase of liquid nitrogen:
13 months.
After thawing:
The product should be administered immediately after thawing. In-use storage times and conditions should not exceed 2 hours at room temperature (15°C-25°C).
Do not refreeze.
6.4. Special precautions for storage
Breyanzi must be stored and transported frozen in the vapour phase of liquid nitrogen (≤ -130°C) and must remain frozen until the patient is ready for treatment to ensure viable cells are available for patient administration. Thawed medicinal product should not be refrozen.
For storage conditions after thawing of the medicinal product, see section 6.3.
6.5. Nature and contents of container
Breyanzi is supplied in cryopreservation vials made of cyclic olefin copolymer. Each 5 mL vial contains 4.6 mL cell dispersion.
The CAR-positive viable T-cells (CD8+ cell component or CD4+ cell component) are presented in individual cartons containing up to 4 vials of each component, depending upon the cryopreserved drug product CAR-positive viable T-cell concentration.
The cartons of CD8+ cell component and CD4+ cell component are contained in a single outer carton.
6.6. Special precautions for disposal and other handling
Precautions to be taken before handling or administering the medicinal product:
- Breyanzi must be transported within the treatment centre in closed, break-proof, leak-proof containers.
- This medicinal product contains human blood cells. Healthcare professionals handling Breyanzi should take appropriate precautions (wearing gloves, protective clothing and eye protection) to avoid potential transmission of infectious diseases.
Preparation prior to administration:
Before thawing the vials:
- Confirm the patient's identity with the patient identifiers on the shipper.
- Breyanzi is composed of CAR-positive viable T cells formulated as separate CD8+ and CD4+ cell components; there is a separate release for infusion certificate (RfIC) for each cell component. Read the RfIC (affixed inside the shipper) for information on the number of syringes you will need and the volume to be administered of the CD8+ and CD4+ cell components (syringe labels are provided with the RfIC).
- Confirm the infusion time in advance and adjust the start time of Breyanzi thaw such that it will be available for infusion when the patient is ready.
Note: Once the vials of CAR-positive viable T-cells (CD8+ cell and CD4+ cell components) are removed from frozen storage, the thaw must be carried to completion and the cells administered within 2 hours.
Thawing the vials:
- Confirm the patient's identity with the patient identifiers on the outer carton and release for infusion certificate (RfIC).
- Remove the CD8+ cell component carton and CD4+ cell component carton from the outer carton.
- Open each inner carton and visually inspect the vial(s) for damage. If the vials are damaged, contact the company.
- Carefully remove the vials from the cartons, place vials on a protective barrier pad, and thaw at room temperature. Thaw all vials at the same time. Take care to keep the CD8+ and CD4+ cell components separate.
Dose preparation:
- Based on the concentration of CAR-positive viable T cells for each component, more than one vial of each of the CD8+ and CD4+ cell components may be required to complete a dose. A separate syringe should be prepared for each CD8+ or CD4+ cell component vial received.
Note: The volume to be drawn up and infused may differ for each component.
- Each 5 mL vial contains a total extractable volume of 4.6 mL of CD8+ or CD4+ cell component T-cells. The release for infusion certificate (RfIC) for each component indicates the volume (mL) of cells to be drawn up into each syringe. Use the smallest Luer-lock tip syringe necessary (1 mL to 5 mL) to draw up the specified volume from each vial. A 5 mL syringe should not be used for volumes less than 3 mL.
- Prepare the syringe(s) of the CD8+ cell component first. Confirm that the patient identifiers on the CD8+ cell component syringe label match the patient identifiers on the CD8+ cell component vial label. Affix the CD8+ cell component syringe labels to the syringe(s) prior to pulling the required volume into the syringe(s).
- Repeat the process for the CD4+ cell component.
Note: It is important to confirm that the volume drawn up for each cell component matches the volume specified in the respective release for infusion certificate (RfIC).
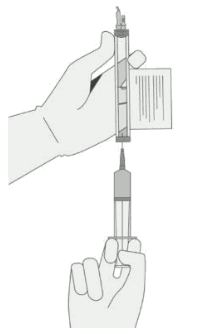
Withdrawal of the required volume of cells from each vial into a separate syringe should be carried out using the following instructions:
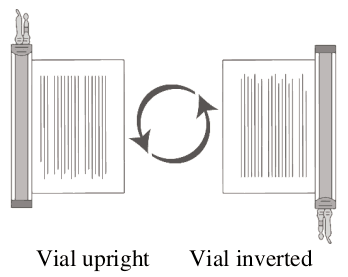
1. Hold the thawed vial(s) upright and gently invert the vial(s) to mix the cell product. If any clumping is apparent, continue to invert the vial(s) until clumps have dispersed and cells appear to be evenly resuspended.
2. Visually inspect the thawed vial(s) for damage or leaks. Do not use if the vial is damaged or if the clumps do not disperse; contact the company. The liquid in the vials should be slightly opaque to opaque, colourless to yellow, or brownish-yellow.
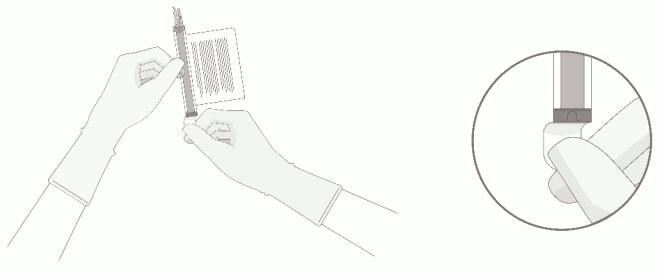
3. Remove the polyaluminium cover (if present) from the bottom of the vial and swab the septum with an alcohol wipe. Allow to air dry before proceeding.
NOTE: The absence of the polyaluminium cover does not impact the sterility of the vial.
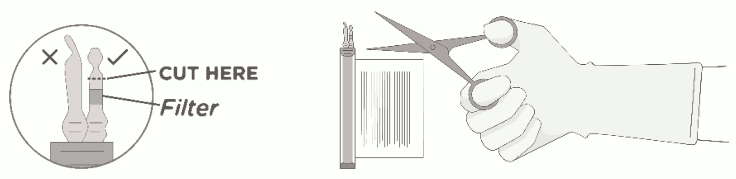
4. Keeping the vial(s) upright, cut the seal on the tubing line on the top of the vial immediately above the filter to open the air vent on the vial.
NOTE: Be careful to select the correct tubing line with the filter. Cut ONLY the tubing with a filter.
5. Hold a 20 gauge, 1-1 ½ inch needle, with the opening of the needle tip away from the retrieval port septum.
a. Insert the needle into the septum at a 45°-60° angle to puncture the retrieval port septum.
b. Increase the angle of the needle gradually as the needle enters the vial.
6. WITHOUT drawing air into the syringe, slowly withdraw the target volume (as specified in the release for infusion certificate, RfIC).
7. Carefully inspect the syringe for signs of debris prior to proceeding. If there is debris, contact the company.
8. Verify that the volume of CD8+/CD4+ cell component matches the volume specified for the relevant component in the release for infusion certificate (RfIC).
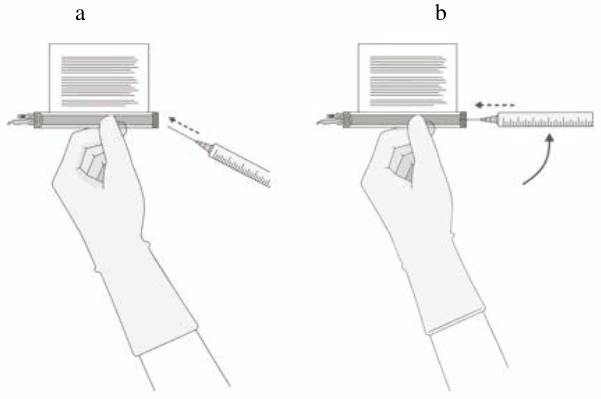
Once the volume is verified, shift the vial and syringe to a horizontal position, and remove the syringe/needle from the vial.
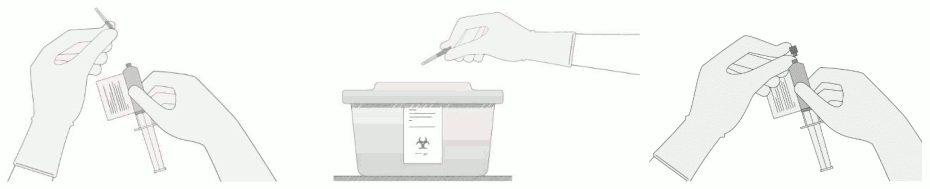
Carefully detach the needle from the syringe and cap the syringe.
9. Continue to keep the vial horizontal and return it to the carton to avoid leaking from the vial.
10. Dispose of any unused portion of Breyanzi.
Administration:
For additional information on administration, see section 4.2.
- Use intravenous sodium chloride 9 mg/mL (0.9%) solution for injection to flush all the infusion tubing prior to and after each CD8+ or CD4+ cell component administration.
- Administer the CD8+ cell component first. The entire volume of the CD8+ cell component is administered intravenously at an infusion rate of approximately 0.5 mL/minute, using the closest port or Y-arm (piggyback).
- If more than one syringe is required for a full dose of the CD8+ cell component, administer the volume in each syringe consecutively without any time between administering the contents of the syringes (unless there is a clinical reason to hold the dose, e.g., infusion reaction). After the CD8+ cell component has been administered, flush the tubing with sodium chloride 9 mg/mL (0.9%) solution for injection.
- Administer the CD4+ cell component immediately after administration of the CD8+ cell component is complete, using the same steps and infusion rate described for the CD8+ cell component. Following administration of the CD4+ cell component, flush the tubing with sodium chloride 9 mg/mL (0.9%) solution for injection, using enough flush to clear the tubing and the length of the IV catheter. The time for infusion will vary and will usually be less than 15 minutes for each component.
Measures to take in case of accidental exposure:
- In case of accidental exposure local guidelines on handling of human derived material must be followed. Work surfaces and materials which have potentially been in contact with Breyanzi must be decontaminated with appropriate disinfectant.
Precautions to be taken for the disposal of the medicinal product:
- Unused medicinal product and all material that has been in contact with Breyanzi (solid and liquid waste) should be handled and disposed of as potentially infectious waste in accordance with local guidelines on handling of human-derived material.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.