CAMPTOSAR Solution for injection Ref.[49708] Active ingredients: Irinotecan
Source: FDA, National Drug Code (US) Revision Year: 2022
Product description
CAMPTOSAR Injection (irinotecan hydrochloride injection) is an antineoplastic agent of the topoisomerase I inhibitor class.
CAMPTOSAR is supplied as a sterile, pale yellow, clear, aqueous solution. Each milliliter of solution contains 20 mg of irinotecan hydrochloride (on the basis of the trihydrate salt), 45 mg of sorbitol, NF, and 0.9 mg of lactic acid, USP. The pH of the solution has been adjusted to 3.5 (range, 3.0 to 3.8) with sodium hydroxide or hydrochloric acid. CAMPTOSAR is intended for dilution with 5% Dextrose Injection, USP (D5W), or 0.9% Sodium Chloride Injection, USP, prior to intravenous infusion. The preferred diluent is 5% Dextrose Injection, USP.
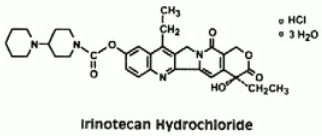
Irinotecan hydrochloride is a semisynthetic derivative of camptothecin, an alkaloid extract from plants such as Camptotheca acuminata or is chemically synthesized.
The chemical name is (S)-4,11-diethyl-3,4,12,14-tetrahydro-4-hydroxy-3,14-dioxo1H-pyrano[3', 4':6,7]-indolizino[1,2-b]quinolin-9-yl-[1,4'bipiperidine]-1'-carboxylate, monohydrochloride, trihydrate. Its empirical formula is C33H38N4O6∙HCl∙3H2O and molecular weight is 677.19. It is slightly soluble in water and organic solvents. Its structural formula is as follows:
| Dosage Forms and Strengths |
|---|
|
Injection: 40 mg/2 mL (20 mg/mL), 100 mg/5 mL (20 mg/mL), and 300 mg/15 mL (20 mg/mL) sterile, pale yellow, clear, aqueous solution in a single-dose vial. |
| How Supplied |
|---|
|
CAMPTOSAR (irinotecan hydrochloride) Injection is available as a sterile, pale yellow, clear, aqueous solution in a vial packaged within a carton in the following packaging configurations: Single-dose amber colored polypropylene CYTOSAFE vial presentations:
|
Drugs
| Drug | Countries | |
|---|---|---|
| CAMPTOSAR | Brazil, Ecuador, Mexico, New Zealand, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.