CARDENE IV Solution for injection Ref.[27508] Active ingredients: Nicardipine
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
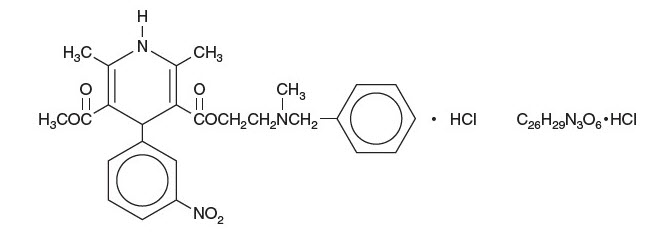
Cardene (nicardipine hydrochloride) is a calcium ion influx inhibitor (slow channel blocker or calcium channel blocker). Cardene I.V. Premixed Injection for intravenous administration contains 20 mg (0.1 mg/mL) of nicardipine hydrochloride per 200 mL in either dextrose or sodium chloride or 40 mg (0.2 mg/mL) of nicardipine hydrochloride per 200 mL in sodium chloride. Nicardipine hydrochloride is a dihydropyridine derivative with IUPAC (International Union of Pure and Applied Chemistry) chemical name (±)2(benzyl-methyl amino) ethyl methyl 1,4-dihydro2,6-dimethyl-4-(m-nitrophenyl)-3,5-pyridinedicarboxylate monohydrochloride and has the following structure:
Nicardipine hydrochloride is a greenish-yellow, odorless, crystalline powder that melts at about 169ºC. It is freely soluble in chloroform, methanol, and glacial acetic acid, sparingly soluble in anhydrous ethanol, slightly soluble in n-butanol, water, 0.01 M potassium dihydrogen phosphate, acetone, and dioxane, very slightly soluble in ethyl acetate, and practically insoluble in benzene, ether, and hexane. It has a molecular weight of 515.99.
Cardene I.V. Premixed Injection is available as a ready-to-use sterile, non-pyrogenic, clear, colorless to yellow, iso-osmotic solution for intravenous administration in a 200 mL GALAXY container with 20 mg (0.1 mg/mL) or 40 mg (0.2 mg/mL) nicardipine hydrochloride in either dextrose or sodium chloride.
Cardene I.V. Premixed Injection in 4.8% Dextrose
20 mg in 200 mL (0.1 mg/mL)
Each mL contains 0.1 mg nicardipine hydrochloride, 48 mg dextrose hydrous, USP, 0.0192 mg citric acid, anhydrous, USP, and 1.92 mg sorbitol, NF. Hydrochloric acid and/or sodium hydroxide may have been added to adjust pH to 3.7 to 4.7.
Cardene I.V. Premixed Injection in 0.86% Sodium Chloride
20 mg in 200 mL (0.1 mg/mL)
Each mL contains 0.1 mg nicardipine hydrochloride, 8.6 mg sodium chloride, USP, 0.0192 mg citric acid, anhydrous, USP, and 1.92 mg sorbitol, NF. Hydrochloric acid and/or sodium hydroxide may have been added to adjust pH to 3.7 to 4.7.
Cardene I.V. Premixed Injection in 0.83% Sodium Chloride
40 mg in 200 mL (0.2 mg/mL)
Each mL contains 0.2 mg nicardipine hydrochloride, 8.3 mg sodium chloride, USP, 0.0384 mg citric acid, anhydrous, USP, and 3.84 mg sorbitol, NF. Hydrochloric acid and/or sodium hydroxide may have been added to adjust pH to 3.7 to 4.7.
The GALAXY container is fabricated from multilayered plastic (PL 2501). Solutions are in contact with the polyethylene layer of the container and can leach out certain chemical components of the plastic in very small amounts within the expiration period. The suitability and safety of the plastic have been confirmed in tests in animals according to the USP biological tests for plastic containers, as well as by tissue culture toxicity studies.
| Dosage Forms and Strengths |
|---|
|
Injection: 200 mL nicardipine (0.1 mg/mL) in either dextrose (4.8%) or sodium chloride (0.86%) as a clear, colorless solution, ready-to-use, iso-osmotic solution in a single use GALAXY container Injection: 200 mL nicardipine (0.2 mg/mL) in sodium chloride (0.83%) as a clear, colorless solution, ready-to-use, iso-osmotic solution in a single use GALAXY container |
| How Supplied | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Cardene I.V. Premixed Injection is supplied as a single-use, ready-to-use, iso-osmotic solution for intravenous administration in a 200 mL GALAXY container with 20 mg (0.1 mg/mL) nicardipine hydrochloride in either dextrose or sodium chloride or 40 mg (0.2 mg/mL) nicardipine hydrochloride in sodium chloride.
Manufactured and Marketed by: Baxter Logo, Baxter Healthcare Corporation, Deerfield, IL 60015 USA |
Drugs
| Drug | Countries | |
|---|---|---|
| CARDENE | Netherlands, United Kingdom, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.