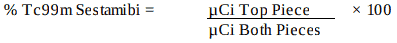CARDIOLITE Powder for solution, lyophilized Ref.[49661] Active ingredients: Technetium ⁹⁹ᵐTc sestamibi
Source: FDA, National Drug Code (US) Revision Year: 2019
1. Indications and Usage
Myocardial Imaging
CARDIOLITE, Kit for the Preparation of Technetium Tc99m Sestamibi for Injection, is a myocardial perfusion agent that is indicated for detecting coronary artery disease by localizing myocardial ischemia (reversible defects) and infarction (non-reversible defects), in evaluating myocardial function and developing information for use in patient management decisions. CARDIOLITE evaluation of myocardial ischemia can be accomplished with rest and cardiovascular stress techniques (e.g., exercise or pharmacologic stress in accordance with the pharmacologic stress agent’s labeling).
It is usually not possible to determine the age of a myocardial infarction or to differentiate a recent myocardial infarction from ischemia.
Breast Imaging
MIRALUMA, Kit for the Preparation of Technetium Tc99m Sestamibi for Injection, is indicated for planar imaging as a second line diagnostic drug after mammography to assist in the evaluation of breast lesions in patients with an abnormal mammogram or a palpable breast mass.
MIRALUMA is not indicated for breast cancer screening, to confirm the presence or absence of malignancy, and it is not an alternative to biopsy.
2. Dosage and Administration
For Myocardial Imaging: The suggested dose range for I.V. administration of CARDIOLITE in a single dose to be employed in the average patient (70 Kg) is 370-1110 MBq (10-30 mCi).
For Breast Imaging: The recommended dose range for I.V. administration of MIRALUMA is a single dose of 740-1110 MBq (20-30 mCi).
2.1 Image Acquisition
Breast Imaging
It is recommended that images are obtained with a table overlay to separate breast tissue from the myocardium and liver, and to exclude potential activity that may be present in the opposite breast. For lateral images, position the patient prone with the isolateral arm comfortably above the head, shoulders flat against the table, head turned to the side and relaxed, with the breast imaged pendent through an overlay cutout. The breast should not be compressed on the overlay. For anterior images, position the patient supine with both arms behind the head. For either lateral or anterior images, shield the chest and abdominal organs, or remove them from the field of view.
For complete study, sets of images should be obtained five minutes after the injection, and in the following sequence:
Beginning five minutes after the injection of Technetium Tc99m Sestamibi:
- ten-minute lateral image of breast with abnormality
- ten-minute lateral image of contralateral breast
- ten-minute anterior image of both breasts
2.2 Radiation Dosimetry
The radiation doses to organs and tissues of an average patient (70 Kg) per 1110 MBq (30 mCi) of Technetium Tc99m Sestamibi injected intravenously are shown in Table 1.0.
Table 1.0. Radiation Absorbed Doses from Tc99m Sestamibi:
| Estimated Radiation Absorbed Dose | ||||
|---|---|---|---|---|
| REST | ||||
| 2.0 hour void | 4.8 hour void | |||
| Organ | rads/ 30 mCi | mGy/ 1110 MBq | rads/ 30 mCi | mGy/ 1110 MBq |
| Breasts | 0.2 | 2.0 | 0.2 | 1.9 |
| Gallbladder Wall | 2.0 | 20.0 | 2.0 | 20.0 |
| Small Intestine | 3.0 | 30.0 | 3.0 | 30.0 |
| Upper Large Intestine Wall | 5.4 | 55.5 | 5.4 | 55.5 |
| Lower Large Intestine Wall | 3.9 | 40.0 | 4.2 | 41.1 |
| Stomach Wall | 0.6 | 6.1 | 0.6 | 5.8 |
| Heart Wall | 0.5 | 5.1 | 0.5 | 4.9 |
| Kidneys | 2.0 | 20.0 | 2.0 | 20.0 |
| Liver | 0.6 | 5.8 | 0.6 | 5.7 |
| Lungs | 0.3 | 2.8 | 0.3 | 2.7 |
| Bone Surfaces | 0.7 | 6.8 | 0.7 | 6.4 |
| Thyroid | 0.7 | 7.0 | 0.7 | 7.0 |
| Ovaries | 1.5 | 15.5 | 1.6 | 15.5 |
| Testes | 0.3 | 3.4 | 0.4 | 3.9 |
| Red Marrow | 0.5 | 5.1 | 0.5 | 5.0 |
| Urinary Bladder Wall | 2.0 | 20.0 | 4.2 | 41.1 |
| Total Body | 0.5 | 4.8 | 0.5 | 4.8 |
| STRESS | ||||
|---|---|---|---|---|
| 2.0 hour void | 4.8 hour void | |||
| Organ | rads/ 30 mCi | mGy/ 1110 MBq | rads/ 30 mCi | mGy/ 1110 MBq |
| Breasts | 0.2 | 2.0 | 0.2 | 1.8 |
| Gallbladder Wall | 2.8 | 28.9 | 2.8 | 27.8 |
| Small Intestine | 2.4 | 24.4 | 2.4 | 24.4 |
| Upper Large Intestine Wall | 4.5 | 44.4 | 4.5 | 44.4 |
| Lower Large Intestine Wall | 3.3 | 32.2 | 3.3 | 32.2 |
| Stomach Wall | 0.6 | 5.3 | 0.5 | 5.2 |
| Heart Wall | 0.5 | 5.6 | 0.5 | 5.3 |
| Kidneys | 1.7 | 16.7 | 1.7 | 16.7 |
| Liver | 0.4 | 4.2 | 0.4 | 4.1 |
| Lungs | 0.3 | 2.6 | 0.2 | 2.4 |
| Bone Surfaces | 0.6 | 6.2 | 0.6 | 6.0 |
| Thyroid | 0.3 | 2.7 | 0.2 | 2.4 |
| Ovaries | 1.2 | 12.2 | 1.3 | 13.3 |
| Testes | 0.3 | 3.1 | 0.3 | 3.4 |
| Red Marrow | 0.5 | 4.6 | 0.5 | 4.4 |
| Urinary Bladder Wall | 1.5 | 15.5 | 3.0 | 30.0 |
| Total Body | 0.4 | 4.2 | 0.4 | 4.2 |
Radiation dosimetry calculations performed by Radiation Internal Dose Information Center, Oak Ridge Institute for Science and Education, PO Box 117, Oak Ridge, TN 37831-0117.
2.3 Instructions For Preparation
Preparation of the Technetium Tc99m Sestamibi from the Kit for the Preparation of Technetium Tc99m Sestamibi is done by the following aseptic procedure:
General Procedure
- Prior to adding the Sodium Pertechnetate Tc99m Injection to the vial, inspect the vial carefully for the presence of damage, particularly cracks, and do not use the vial if found. Tear off a radiation symbol and attach it to the neck of the vial.
- Waterproof gloves should be worn during the preparation procedure. Remove the plastic disc from the vial and swab the top of the vial closure with alcohol to sanitize the surface.
Boiling Water Bath Procedure
- Place the vial in a suitable radiation shield with a fitted radiation cap.
- With a sterile shielded syringe, aseptically obtain additive-free, sterile, non-pyrogenic Sodium Pertechnetate Tc99m Injection [925-5550 MBq, (25-150 mCi)] in approximately 1 to 3 mL.
- Aseptically add the Sodium Pertechnetate Tc99m Injection to the vial in the lead shield. Without withdrawing the needle, remove an equal volume of headspace to maintain atmospheric pressure within the vial.
- Shake vigorously, about 5 to 10 quick upward-downward motions.
- Remove the vial from the lead shield and place upright in an appropriately shielded and contained boiling water bath, such that the vial is suspended above the bottom of the bath, and boil for 10 minutes. Timing for 10 minutes is begun as soon as the water begins to boil again. Do not allow the boiling water to come in contact with the aluminum crimp.
- Remove the vial from the water bath, place in the lead shield and allow to cool for fifteen minutes.
Recon-o-Stat (thermal cycler) Procedure
- Place the vial in the thermal cycler radiation shield.
- With a sterile shielded syringe, aseptically obtain additive-free, sterile, non-pyrogenic Sodium Pertechnetate Tc99m Injection [925-5550 MBq, (25-150 mCi)] in approximately 1 to 3 mL.
- Aseptically add the Sodium Pertechnetate Tc99m Injection to the vial in the lead shield. Without withdrawing the needle, remove an equal volume of headspace to maintain atmospheric pressure within the vial.
- Shake vigorously, about 5 to 10 quick upward-downward motions.
- Place shield on sample block. While slightly pressing downward, give the shield a quarter turn to make certain there is a firm fit between the shield and the sample block.
- Press the proceed button to initiate the program (the thermal cycler automatically heats & cools the vial and contents). Please see the Recon-o-Stat Instruction Manual for further details.
General Procedure (cont.)
- Using proper shielding, the vial contents should be visually inspected. Use only if the solution is clear and free of particulate matter and discoloration.
- Assay the reaction vial using a suitable radioactivity calibration system. Record the Technetium Tc99m concentration, total volume, assay time and date, expiration time and lot number on the vial shield label and affix the label to the shield.
- Store the reaction vial containing the Technetium Tc99m Sestamibi at 15° to 25°C (59°-77°F) until use; at such time the product should be aseptically withdrawn. Technetium Tc99m Sestamibi should be used within six hours of preparation. The vial contains no preservative.
Note: Adherence to the above product reconstitution instructions is recommended.
The potential for cracking and significant contamination exists whenever vials containing radioactive material are heated.
Product should be used within 6 hours after preparation.
Final product with radiochemical purity of at least 90% was used in the clinical trials that established safety and effectiveness. The radiochemical purity was determined by the following method.
2.4 Determination of Radiochemical Purity in Technetium Tc99m Sestamibi
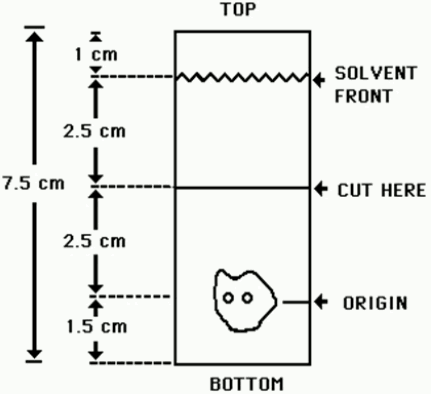
- Obtain a Baker-Flex Aluminum Oxide coated, plastic TLC plate, #1 B-F, pre-cut to 2.5 cm × 7.5 cm.
- Dry the plate or plates at 100°C for 1 hour and store in a desiccator. Remove pre-dried plate from the desiccator just prior to use.
- Apply 1 drop of ethanol?footnote? using a 1 mL syringe with a 22-26 gauge needle, 1.5 cm from the bottom of the plate. THE SPOT SHOULD NOT BE ALLOWED TO DRY.
- Add 2 drops of Technetium Tc99m Sestamibi solution, side by side on top of the ethanol1 spot. Return the plate to a desiccator and allow the sample spot to dry (typically 15 minutes).
- The TLC tank is prepared by pouring ethanol1 to a depth of 3-4 mm. Cover the tank and let it equilibrate for ~10 minutes.
- Develop the plate in the covered TLC tank in ethanol1 for a distance of 5 cm from the point of application.
- Cut the TLC plate 4 cm from the bottom and measure the Tc99m activity in each piece by appropriate radiation detector.
- Calculate the % Tc99m Sestamibi as:
Figure 1.0 TLC Plate Diagram:
1 The ethanol used in this procedure should be 95% or greater. Absolute ethanol (99%) should remain at ≥95% ethanol content for one week after opening if stored tightly capped, in a cool dry place.
10. Overdosage
The clinical consequences of overdosing with CARDIOLITE are not known.
16.2. Storage and Handling
The contents of the vial are lyophilized and stored under nitrogen. Store at 15-25°C (59-77°F) before and after reconstitution.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.