CEDAX Capsule / Oral suspension Ref.[50729] Active ingredients: Naproxen
Source: FDA, National Drug Code (US) Revision Year: 2010
Product description
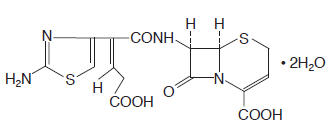
CEDAX (ceftibuten capsules) and (ceftibuten for oral suspension) contain the active ingredient ceftibuten as ceftibuten dihydrate. Ceftibuten dihydrate is a semisynthetic cephalosporin antibiotic for oral administration. Chemically, it is (+)-(6R,7R)-7-[(Z)-2-(2-Amino-4-thiazolyl)-4-carboxycrotonamido]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, dihydrate. Its molecular formula is C15H14N4O6S2•2H2O. Its molecular weight is 446.43 as the dihydrate.
Ceftibuten dihydrate has the following structural formula:
CEDAX Capsules contain ceftibuten dihydrate equivalent to 400 mg of ceftibuten. Inactive ingredients contained in the capsule formulation include: magnesium stearate, microcrystalline cellulose, and sodium starch glycolate. The capsule shell and/or band contains gelatin, sodium lauryl sulfate, titanium dioxide, and polysorbate 80. The capsule shell may also contain benzyl alcohol, sodium propionate, edetate calcium disodium, butylparaben, propylparaben, and methylparaben.
CEDAX Oral Suspension after reconstitution contains ceftibuten dihydrate equivalent to 90 mg of ceftibuten per 5 mL. CEDAX Oral Suspension is cherry flavored and contains the inactive ingredients: cherry flavoring, polysorbate 80, silicon dioxide, simethicone, sodium benzoate, sucrose (approximately 1 g/5 mL), titanium dioxide, and xanthan gum.
| How Supplied |
|---|
|
CEDAX Capsules, containing 400 mg of ceftibuten (as ceftibuten dihydrate) are white, opaque capsules imprinted with the product name and strength, are available as follows:
Sciele Pharma, Inc., Atlanta, GA 30328 Manufactured by Schering Corporation CED01PIB02 Distributed by Sciele Pharma, Inc. |
Drugs
| Drug | Countries | |
|---|---|---|
| CEDAX | Nigeria |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.