CEFPODOXIME PROXETIL Granule, for suspension Ref.[50296] Active ingredients: Cefpodoxime
Source: FDA, National Drug Code (US) Revision Year: 2019
Product description
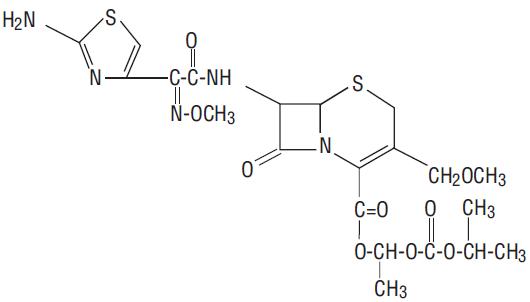
Cefpodoxime proxetil is an orally administered, extended spectrum, semi-synthetic antibiotic of the cephalosporin class. The chemical name is (RS)-1(isopropoxycarbonyloxy) ethyl (+)-(6R,7R)-7-[2-(2-amino-4-thiazolyl)-2-{(Z)methoxyimino}acetamido]-3-methoxymethyl-8-oxo-5-thia-1-azabicyclo [4.2.0]oct-2-ene-2-carboxylate.
Its molecular formula is C21H27N5O9S2 and its structural formula is represented below:
The molecular weight of cefpodoxime proxetil is 557.6.
Cefpodoxime proxetil is a prodrug; its active metabolite is cefpodoxime. All doses of cefpodoxime proxetil in this insert are expressed in terms of the active cefpodoxime moiety. The drug is supplied as flavoured granules for oral suspension.
Each 5 mL of cefpodoxime proxetil for oral suspension USP contains cefpodoxime proxetil USP equivalent to 50 mg or 100 mg of cefpodoxime activity after constitution and the following inactive ingredients: lactose monohydrate, corn starch, croscarmellose sodium, ferric oxide yellow, hydroxypropyl cellulose, microcrystalline cellulose and carboxymethyl cellulose sodium, colloidal silicon dioxide, citric acid anhydrous, sodium citrate, sodium benzoate, sucrose, and citron & vanille flavorings.
| How Supplied |
|---|
|
Cefpodoxime Proxetil for Oral Suspension, USP provides the equivalent of 50 mg or 100 mg cefpodoxime per 5 mL suspension (when constituted as directed) and is available as off-white colored granular powder in the following sizes: 50 mg/5 mL: 50-mL suspension NDC 65862-140-50 100 mg/5 mL: 50-mL suspension NDC 65862-141-50 Distributed by: Aurobindo Pharma USA, Inc., 279 Princeton-Hightstown Road, East Windsor, NJ 08520 Manufactured by: Aurobindo Pharma Limited, Hyderabad-500 038, India |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.