CEREBYX Solution for injection Ref.[10769] Active ingredients:
Source: FDA, National Drug Code (US) Revision Year: 2021
12.1. Mechanism of Action
Fosphenytoin is a prodrug of phenytoin and accordingly, its anticonvulsant effects are attributable to phenytoin. The precise mechanism by which phenytoin exerts its therapeutic effect has not been established but is thought to involve the voltage-dependent blockade of membrane sodium channels resulting in a reduction in sustained high-frequency neuronal discharges.
12.3. Pharmacokinetics
Fosphenytoin
Absorption
Intravenous
When CEREBYX is administered by IV infusion, maximum plasma fosphenytoin concentrations are achieved at the end of the infusion.
Intramuscular
Fosphenytoin is completely bioavailable following IM administration of CEREBYX. Peak concentrations occur at approximately 30 minutes postdose. Plasma fosphenytoin concentrations following IM administration are lower but more sustained than those following IV administration due to the time required for absorption of fosphenytoin from the injection site.
Distribution
Fosphenytoin is extensively bound (95% to 99%) to human plasma proteins, primarily albumin. Binding to plasma proteins is saturable with the result that the percent bound decreases as total fosphenytoin concentrations increase. Fosphenytoin displaces phenytoin from protein binding sites. The volume of distribution of fosphenytoin increases with CEREBYX dose and rate, and ranges from 4.3 to 10.8 liters.
Elimination
The conversion half-life of fosphenytoin to phenytoin is approximately 15 minutes.
Metabolism
Following parenteral administration of CEREBYX, fosphenytoin is converted to the anticonvulsant phenytoin. The mechanism of fosphenytoin conversion has not been determined, but phosphatases probably play a major role. Fosphenytoin is metabolized to phenytoin, phosphate, and formate. For every mmol of fosphenytoin administered, one mmol of phenytoin is produced. The hydrolysis of fosphenytoin to phenytoin yields two metabolites, phosphate and formaldehyde. Formaldehyde is subsequently converted to formate, which is in turn metabolized via a folate dependent mechanism. Although phosphate and formaldehyde (formate) have potentially important biological effects, these effects typically occur at concentrations considerably in excess of those obtained when CEREBYX is administered under conditions of use recommended in this labeling.
Excretion
Fosphenytoin is not excreted in urine.
Phenytoin (after CEREBYX administration)
In general, IM administration of CEREBYX generates systemic phenytoin concentrations that are similar enough to oral phenytoin sodium to allow essentially interchangeable use. The pharmacokinetics of fosphenytoin following IV administration of CEREBYX, however, are complex, and when used in an emergency setting (e.g., status epilepticus), differences in rate of availability of phenytoin could be critical. Studies have therefore empirically determined an infusion rate for CEREBYX that gives a rate and extent of phenytoin systemic availability similar to that of a 50 mg/min phenytoin sodium infusion. A dose of 15 to 20 mg PE/kg of CEREBYX infused at 100 to 150 mg PE/min yields plasma free phenytoin concentrations over time that approximate those achieved when an equivalent dose of phenytoin sodium (e.g., parenteral DILANTIN) is administered at 50 mg/min [see Dosage and Administration (2.3) and Warnings and Precautions (5.2)].
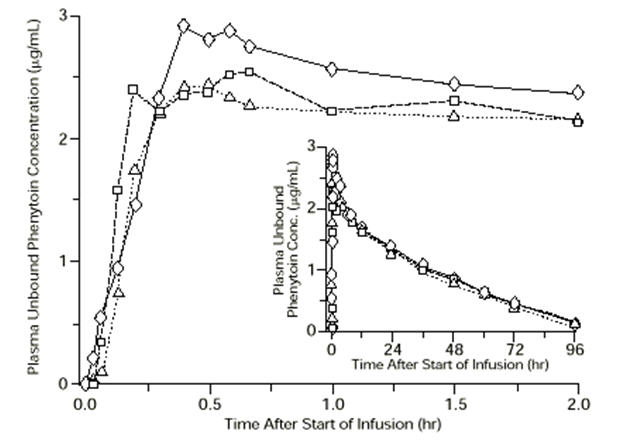
Figure 1. Mean plasma unbound phenytoin concentrations following IV administration of 1200 mg PE CEREBYX infused at 100 mg PE/min (triangles) or 150 mg PE/min (squares) and 1200 mg Dilantin infused at 50 mg/min (diamonds) to healthy subjects (N=12). Inset shows time course for the entire 96-hour sampling period:
Following administration of single IV CEREBYX doses of 400 to 1200 mg PE, mean maximum total phenytoin concentrations increase in proportion to dose, but do not change appreciably with changes in infusion rate. In contrast, mean maximum unbound phenytoin concentrations increase with both dose and rate.
Absorption
Fosphenytoin is completely converted to phenytoin following IV administration, with a half-life of approximately 15 minutes. Fosphenytoin is also completely converted to phenytoin following IM administration and plasma total phenytoin concentrations peak in approximately 3 hours.
Distribution
Phenytoin is highly bound to plasma proteins, primarily albumin, although to a lesser extent than fosphenytoin. In the absence of fosphenytoin, approximately 12% of total plasma phenytoin is unbound over the clinically relevant concentration range. However, fosphenytoin displaces phenytoin from plasma protein binding sites. This increases the fraction of phenytoin unbound (up to 30% unbound) during the period required for conversion of fosphenytoin to phenytoin (approximately 0.5 to 1 hour postinfusion).
Elimination
Mean total phenytoin half-life values (12.0 to 28.9 hr) following CEREBYX administration at these doses are similar to those after equal doses of parenteral Dilantin and tend to be greater at higher plasma phenytoin concentrations.
Metabolism
Phenytoin derived from administration of CEREBYX is extensively metabolized in the liver by the cytochrome P450 enzyme CYP2C9 and to a lesser extent by CYP2C19. Phenytoin hepatic metabolism is saturable, and following administration of single IV CEREBYX doses of 400 to 1200 mg PE, total and unbound phenytoin AUC values increase disproportionately with dose.
Excretion
Phenytoin derived from administration of CEREBYX is excreted in urine primarily as 5-(p-hydroxyphenyl)-5-phenylhydantoin and its glucuronide; little unchanged phenytoin (1% to 5% of the CEREBYX dose) is recovered in urine.
Specific Populations
Age: Geriatric Population
The effect of age on the pharmacokinetics of fosphenytoin was evaluated in patients 5 to 98 years of age. Patient age had no significant impact on fosphenytoin pharmacokinetics. Phenytoin clearance tends to decrease with increasing age (20% less in patients over 70 years of age relative to that in patients 20 to 30 years of age).
Sex/Race
Gender and race have no significant impact on fosphenytoin or phenytoin pharmacokinetics.
Renal or Hepatic Impairment
Increased fraction of unbound phenytoin (the active metabolite of CEREBYX) in patients with renal or hepatic disease, or in those with hypoalbuminemia has been reported.
Pregnancy
It has been reported in the literature that the plasma clearance of phenytoin (the active metabolite of CEREBYX) generally increased during pregnancy, reached a peak in the third trimester and returned to the level of pre-pregnancy after few weeks or months of delivery [see Dosage and Administration (2.9)].
Drug Interaction Studies
Phenytoin derived from administration of CEREBYX is extensively metabolized in the liver by the cytochrome P450 enzyme CYP2C9 and to a lesser extent by CYP2C19 [see Drug Interactions (7.1, 7.2)]. No drugs are known to interfere with the conversion of fosphenytoin to phenytoin. Conversion could be affected by alterations in the level of phosphatase activity, but given the abundance and wide distribution of phosphatases in the body it is unlikely that drugs would affect this activity enough to affect conversion of fosphenytoin to phenytoin.
The pharmacokinetics and protein binding of fosphenytoin, phenytoin, and diazepam were not altered when diazepam and CEREBYX were concurrently administered in single submaximal doses.
12.5. Pharmacogenomics
CYP2C9 activity is decreased in individuals with genetic variants such as the CYP2C9*2 and CYP2C9*3 alleles. Carriers of variant alleles, resulting in intermediate (e.g., *1/*3, *2/*2) or poor metabolism (e.g., *2/*3, *3/*3) have decreased clearance of phenytoin. Other decreased or nonfunctional CYP2C9 alleles may also result in decreased clearance of phenytoin (e.g., *5, *6, *8, *11).
The prevalence of the CYP2C9 poor metabolizer phenotype is approximately 2–3% in the White population, 0.5–4% in the Asian population, and <1% in the African American population. The CYP2C9 intermediate phenotype prevalence is approximately 35% in the White population, 24% in the African American population, and 15–36% in the Asian population [see Warnings and Precautions (5.4) and Use in Specific Populations (8.7)].
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis [see Warnings and Precautions (5.9)]
The carcinogenic potential of fosphenytoin has not been assessed. In carcinogenicity studies, phenytoin (active metabolite of fosphenytoin) was administered in the diet to mice (10, 25, or 45 mg/kg/day) and rats (25, 50, or 100 mg/kg/day) for 2 years. The incidences of hepatocellular tumors were increased in male and female mice at the highest dose. No increases in tumor incidence were observed in rats. The highest doses tested in these studies were associated with peak plasma phenytoin levels below human therapeutic concentrations.
In carcinogenicity studies reported in the literature, phenytoin was administered in the diet for 2 years at doses up to 600 ppm (approximately 160 mg/kg/day) to mice and up to 2400 ppm (approximately 120 mg/kg/day) to rats. The incidences of hepatocellular tumors were increased in female mice at all but the lowest dose tested. No increases in tumor incidence were observed in rats.
Mutagenesis
An increase in structural chromosome aberrations were observed in cultured V79 Chinese hamster lung cells exposed to fosphenytoin in the presence of metabolic activation. No evidence of mutagenicity was observed in bacteria (Ames test) or Chinese hamster lung cells in vitro, and no evidence for clastogenic activity was observed in an in vivo mouse bone marrow micronucleus assay.
Impairment of Fertility
Fosphenytoin was administered to male and female rats during mating and continuing in females throughout gestation and lactation at doses of 50 mg PE/kg or higher. No effects on fertility were observed in males. In females, altered estrous cycles, delayed mating, prolonged gestation length, and developmental toxicity were observed at all doses, which were associated with maternal toxicity. The lowest dose tested is approximately 40% of the maximum human loading dose on a mg/m² basis.
14. Clinical Studies
Infusion tolerance was evaluated in clinical studies. One double-blind study assessed infusion-site tolerance of equivalent loading doses (15 to 20 mg PE/kg) of CEREBYX infused at 150 mg PE/min or phenytoin infused at 50 mg/min. The study demonstrated better local tolerance (pain and burning at the infusion site), fewer disruptions of the infusion, and a shorter infusion period for CEREBYX-treated patients (Table 8).
Table 8. Infusion Tolerance of Equivalent Loading Doses of IV CEREBYX and IV Phenytoin:
| IV CEREBYX N=90 | IV Phenytoin N=22 | |
|---|---|---|
| Local Intolerance | 9%* | 90% |
| Infusion Disrupted | 21% | 67% |
| Average Infusion Time | 13 min | 44 min |
* Percent of patients
CEREBYX-treated patients, however, experienced more systemic sensory disturbances [see Warnings and Precautions (5.10)]. Infusion disruptions in CEREBYX-treated patients were primarily due to systemic burning, pruritus, and/or paresthesia while those in phenytoin-treated patients were primarily due to pain and burning at the infusion site (see Table 8). In a double-blind study investigating temporary substitution of CEREBYX for oral phenytoin, IM CEREBYX was as well-tolerated as IM placebo. IM CEREBYX resulted in a slight increase in transient, mild to moderate local itching (23% of CEREBYX-treated patients vs 11% of IM placebo-treated patients at any time during the study). This study also demonstrated that equimolar doses of IM CEREBYX may be substituted for oral phenytoin sodium with no dosage adjustments needed when initiating IM or returning to oral therapy. In contrast, switching between IM and oral phenytoin requires dosage adjustments because of slow and erratic phenytoin absorption from muscle.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.