CERVIDIL Vaginal insert Ref.[10770] Active ingredients: Dinoprostone
Source: FDA, National Drug Code (US) Revision Year: 2020
1. Indications and Usage
CERVIDIL is indicated for the initiation and/or continuation of cervical ripening in pregnant women at or near term in whom there is a medical or obstetrical indication for the induction of labor.
2. Dosage and Administration
2.1 Dosage Instructions
Administer one CERVIDIL insert (10 mg) intravaginally for use up to 12 hours (approximately 0.3 mg of dinoprostone is released per hour) [see Dosage and Administration (2.2)].
Monitor uterine activity, fetal status, and the progression of cervical dilatation and effacement with the use of CERVIDIL. Remove CERVIDIL 12 hours after insertion with the onset of active labor, prior to an amniotomy, occurrence of uterine tachysystole, uterine hypersystole/hypertonicity, or fetal distress [see Warnings and Precautions (5.4)]. Remove CERVIDIL at least 30 minutes prior to administering an oxytocic agent [see Warnings and Precautions (5.4) and Drug Interactions (7)].
2.2 Preparation and Administration Instructions
CERVIDIL should be administered only by trained obstetrical personnel in a hospital setting with appropriate obstetrical care facilities.
Preparation and Administration Instructions:
- Keep CERVIDIL frozen until ready for use. Do not warm CERVIDIL prior to vaginal insertion.
- Tear open the individually-wrapped foil package containing CERVIDIL along the tear mark. Never open the package using scissors or other sharp objects because this may damage the knitted polyester retrieval system. Do not cut the retrieval system and do not use CERVIDIL unless its retrieval system is intact.
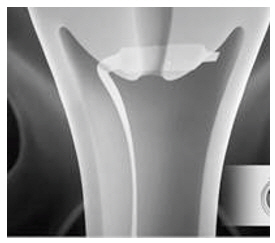
- Immediately after opening the package, insert CERVIDIL transversely, in the posterior fornix of the vagina (see Figure 1). If necessary, use a minimal amount of water-miscible lubricant to assist vaginal insertion. Do not permit excess contact or coating with the lubricant, as this could prevent release of dinoprostone from the vaginal insert. Insertion does not require sterile conditions.
Figure 1. Placement of CERVIDIL in the Posterior Vaginal Fornix:
- Tuck some of the excess retrieval system into the vagina to avoid movement of CERVIDIL away from the proper position; however, leave a small amount of the retrieval system outside the vagina to aid in retrieval.
- Instruct women to remain in a recumbent position during insertion of CERVIDIL and for 2 hours afterward. Women may be ambulatory 2 hours after insertion; however, ensure that the insert remains in place.
2.3 Removal Instructions
- To retrieve, locate the retrieval system and pull it gently until CERVIDIL is fully removed.
- Upon removal of CERVIDIL, it is essential to perform a visual inspection to ensure that the slab has been removed, as it will continue delivering the active ingredient.
16.2. Storage and Handling
Store in a freezer between -20°C and -10°C (-4°F and 14°F). CERVIDIL, enclosed in its aluminum/polyethylene pack, is stable when stored in a freezer for a period of three years. Vaginal inserts exposed to high humidity will absorb moisture from the air and thereby alter the release characteristics of dinoprostone.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.