CIPRALEX Oral drops, solution Ref.[6768] Active ingredients: Escitalopram
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2017 Publisher: H. Lundbeck A/S, Ottilavaj 9, 2500 Valby, Denmark
Therapeutic indications
- Treatment of major depressive episodes.
- Treatment of panic disorder with or without agoraphobia.
- Treatment of social anxiety disorder (social phobia).
- Treatment of generalised anxiety disorder.
- Treatment of obsessive-compulsive disorder.
Posology and method of administration
Safety of daily doses above 20 mg (20 drops) has not been demonstrated.
Cipralex is administered as a single daily dose and may be taken with or without food.
Cipralex oral drops, solution can be mixed with water, orange juice or apple juice.
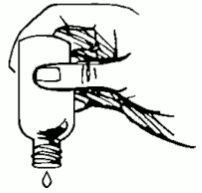
Turn the bottle completely upside down. If no drops come out, tap the bottle lightly to start the flow.
Major depressive episodes
Usual dosage is 10 mg (10 drops) once daily. Depending on individual patient response, the dose may be increased to a maximum of 20 mg (20 drops) daily.
Usually 2-4 weeks are necessary to obtain antidepressant response. After the symptoms resolve, treatment for at least 6 months is required for consolidation of the response.
Panic disorder with or without agoraphobia
An initial dose of 5 mg (5 drops) is recommended for the first week before increasing the dose to 10 mg (10 drops) daily. The dose may be further increased, up to a maximum of 20 mg (20 drops) daily, dependent on individual patient response.
Maximum effectiveness is reached after about 3 months. The treatment lasts several months.
Social anxiety disorder
Usual dosage is 10 mg (10 drops) once daily. Usually 2-4 weeks are necessary to obtain symptom relief. The dose may subsequently, depending on individual patient response, be decreased to 5 mg (5 drops) or increased to a maximum of 20 mg (20 drops) daily.
Social anxiety disorder is a disease with a chronic course, and treatment for 12 weeks is recommended to consolidate response. Long-term treatment of responders has been studied for 6 months and can be considered on an individual basis to prevent relapse; treatment benefits should be re-evaluated at regular intervals.
Social anxiety disorder is a well-defined diagnostic terminology of a specific disorder, which should not be confounded with excessive shyness. Pharmacotherapy is only indicated if the disorder interferes significantly with professional and social activities.
The place of this treatment compared to cognitive behavioural therapy has not been assessed. Pharmacotherapy is part of an overall therapeutic strategy.
Generalised anxiety disorder
Initial dosage is 10 mg (10 drops) once daily. Depending on the individual patient response, the dose may be increased to a maximum of 20 mg (20 drops) daily.
Long-term treatment of responders has been studied for at least 6 months in patients receiving 20 mg (20 drops) daily. Treatment benefits and dose should be re-evaluated at regular intervals (see Section 5.1).
Obsessive-compulsive disorder
Initial dosage is 10 mg (10 drops) once daily. Depending on the individual patient response, the dose may be increased to a maximum of 20 mg (20 drops) daily.
As OCD is a chronic disease, patients should be treated for a sufficient period to ensure that they are symptom free.
Treatment benefits and dose should be re-evaluated at regular intervals (see section 5.1).
Elderly patients (>65 years of age)
Initial dosage is 5 mg (5 drops) once daily. Depending on individual patient response the dose may be increased to 10 mg (10 drops) daily (see section 5.2).
The efficacy of Cipralex in social anxiety disorder has not been studied in elderly patients.
Children and adolescents (<18 years)
Cipralex should not be used in the treatment of children and adolescents under the age of 18 years (see section 4.4).
Reduced renal function
Dosage adjustment is not necessary in patients with mild or moderate renal impairment. Caution is advised in patients with severely reduced renal function (CLCR less than 30 ml/min.) (see section 5.2).
Reduced hepatic function
An initial dose of 5 mg (5 drops) daily for the first two weeks of treatment is recommended in patients with mild or moderate hepatic impairment. Depending on individual patient response, the dose may be increased to 10 mg (10 drops) daily. Caution and extra careful dose titration is advised in patients with severely reduced hepatic function (see section 5.2).
Poor metabolisers of CYP2C19
For patients who are known to be poor metabolisers with respect to CYP2C19, an initial dose of 5 mg (5 drops) daily during the first two weeks of treatment is recommended. Depending on individual patient response, the dose may be increased to 10 mg (10 drops) daily (see section 5.2).
Discontinuation symptoms seen when stopping treatment
Abrupt discontinuation should be avoided. When stopping treatment with escitalopram the dose should be gradually reduced over a period of at least one to two weeks in order to reduce the risk of discontinuation symptoms (see sections 4.4 and 4.8). If intolerable symptoms occur following a decrease in the dose or upon discontinuation of treatment, then resuming the previously prescribed dose may be considered. Subsequently, the physician may continue decreasing the dose, but at a more gradual rate.
Overdose
Toxicity
Clinical data on escitalopram overdose are limited and many cases involve concomitant overdoses of other drugs. In the majority of cases mild or no symptoms have been reported. Fatal cases of escitalopram overdose have rarely been reported with escitalopram alone; the majority of cases have involved overdose with concomitant medications. Doses between 400 and 800 mg of escitalopram alone have been taken without any severe symptoms.
Symptoms
Symptoms seen in reported overdose of escitalopram include symptoms mainly related to the central nervous system (ranging from dizziness, tremor, and agitation to rare cases of serotonin syndrome, convulsion, and coma), the gastrointestinal system (nausea/vomiting), and the cardiovascular system (hypotension, tachycardia, QT interval prolongation, and arrhythmia) and electrolyte/fluid balance conditions (hypokalaemia, hyponatraemia).
Management
There is no specific antidote. Establish and maintain an airway, ensure adequate oxygenation and respiratory function. Gastric lavage and the use of activated charcoal should be considered. Gastric lavage should be carried out as soon as possible after oral ingestion. Cardiac and vital signs monitoring are recommended along with general symptomatic supportive measures.
ECG monitoring is advised in case of overdose in patients with congestive heart failure/bradyarrhythmias, in patients using concomitant medications that prolong the QT interval, or in patients with altered metabolism, e.g. liver impairment.
Shelf life
Shelf life: 3 years.
After opening, the drops should be used within 8 weeks.
Special precautions for storage
After opening the bottle should not be stored above 25°C.
Nature and contents of container
15 ml in a brown glass bottle with dropper applicator (polyethylene), and child-proof screw cap (polypropylene).
Special precautions for disposal and other handling
No special requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.