CLEVIPREX Emulsion for infusion Ref.[27458] Active ingredients: Clevidipine
Source: FDA, National Drug Code (US) Revision Year: 2021
Product description
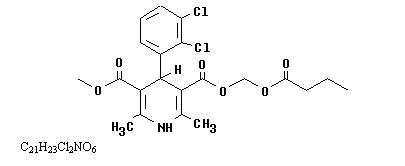
Cleviprex is a sterile, milky-white emulsion containing 0.5 mg/mL of clevidipine suitable for intravenous administration. Clevidipine is a dihydropyridine calcium channel blocker. Chemically, the active substance, clevidipine, is butyroxymethyl methyl 4-(2´,3´-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate. It is a racemic mixture with a molecular weight of 456.3 g/mol. Each enantiomer has equipotent antihypertensive activity.
The structure and formula are:
Clevidipine is practically insoluble in water and is formulated in an oil-in-water emulsion. In addition to the active ingredient, clevidipine, Cleviprex contains soybean oil (200 mg/mL), glycerin (22.5 mg/mL), purified egg yolk phospholipids (12 mg/mL), oleic acid (0.3 mg/mL), disodium edetate (0.05 mg/mL), and sodium hydroxide to adjust pH. Cleviprex has a pH of 6.0–8.0 and is a ready-to-use emulsion.
| Dosage Forms and Strengths |
|---|
|
Cleviprex is a sterile, milky white injectable emulsion for intravenous use, available in the following configurations:
|
| How Supplied |
|---|
|
Cleviprex (clevidipine) injectable emulsion is supplied as a sterile, milky white liquid emulsion product in single-use glass vials at a concentration of 0.5 mg/mL of clevidipine. NDC 10122-610-10: 10 Single Use 50 mL Vials Manufactured by: Fresenius Kabi Austria GmbH, Graz, Austria Marketed by: Chiesi USA, Inc., Cary, NC 27518 |
Drugs
| Drug | Countries | |
|---|---|---|
| CLEVIPREX | Austria, Spain, France, Netherlands, New Zealand, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.