CLIMARA Patch Ref.[10547] Active ingredients: Estradiol
Source: FDA, National Drug Code (US) Revision Year: 2017
12.1. Mechanism of Action
Endogenous estrogens are largely responsible for the development and maintenance of the female reproductive system and secondary sexual characteristics. Although circulating estrogens exist in a dynamic equilibrium of metabolic interconversions, estradiol is the principal intracellular human estrogen and is substantially more potent than its metabolites, estrone and estriol at the receptor level.
The primary source of estrogen in normally cycling adult women is the ovarian follicle, which secretes 70 to 500 mcg of estradiol daily, depending on the phase of the menstrual cycle. After menopause, most endogenous estrogen is produced by conversion of androstenedione, which is secreted by the adrenal cortex, to estrone in the peripheral tissues. Thus, estrone and the sulfate conjugated form, estrone sulfate, are the most abundant circulating estrogens in postmenopausal women.
Estrogens act through binding to nuclear receptors in estrogen-responsive tissues. To date, two estrogen receptors have been identified. These vary in proportion from tissue to tissue.
Circulating estrogens modulate the pituitary secretion of the gonadotropins, luteinizing hormone (LH) and FSH, through a negative feedback mechanism. Estrogens act to reduce the elevated levels of these hormones seen in postmenopausal women.
12.2. Pharmacodynamics
There are no pharmacodynamic data for Climara.
12.3. Pharmacokinetics
Absorption
Transdermal administration of Climara produces mean serum concentrations of estradiol comparable to those produced by premenopausal women in the early follicular phase of the ovulatory cycle. The pharmacokinetics of estradiol following application of the Climara transdermal system were investigated in 197 healthy postmenopausal women in six studies. In five of the studies, the Climara transdermal system was applied to the abdomen, and in a sixth study, application to the buttocks and abdomen were compared.
The Climara transdermal delivery system continuously releases estradiol which is transported across intact skin leading to sustained circulating levels of estradiol during a 7-day treatment period. The systemic availability of estradiol after transdermal administration is about 20 times higher than that after oral administration. This difference is due to the absence of first pass metabolism when estradiol is given by the transdermal route.
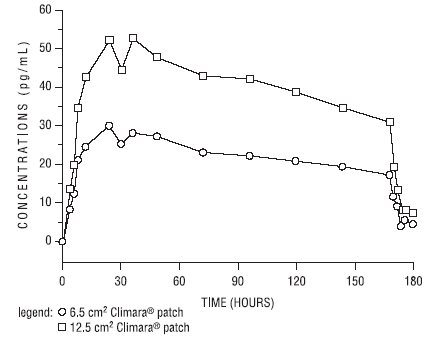
In a bioavailability study, the Climara 6.5 cm² was studied with the Climara 12.5 cm² as reference. The mean estradiol levels in serum from the two sizes are shown in Figure 1.
Figure 1. Mean Serum 17β-Estradiol Concentrations versus Time Profile following Application of a 6.5 cm² Transdermal System and Application of a 12.5 cm² Climara Transdermal System:
Dose proportionality was demonstrated for the Climara 6.5 cm² transdermal system as compared to the Climara 12.5 cm² transdermal system in a 2-week crossover study with a 1-week washout period between the two-transdermal systems in 24 postmenopausal women.
Dose proportionality was also demonstrated for the Climara transdermal system (12.5 cm² and 25 cm²) in a 1-week study conducted in 54 postmenopausal women. The mean steady state levels (Cavg) of the estradiol during the application of Climara 25 cm² and 12.5 cm² on the abdomen were about 80 and 40 pg/mL, respectively.
In a 3-week multiple application study in 24 postmenopausal women, the 25 cm² Climara transdermal system produced average peak estradiol concentrations (Cmax) of approximately 100 pg/mL. Trough values at the end of each wear interval (Cmin) were approximately 35 pg/mL. Nearly identical serum curves were seen each week, indicating little or no accumulation of estradiol in the body. Serum estrone peak and trough levels were 60 and 40 pg/mL, respectively.
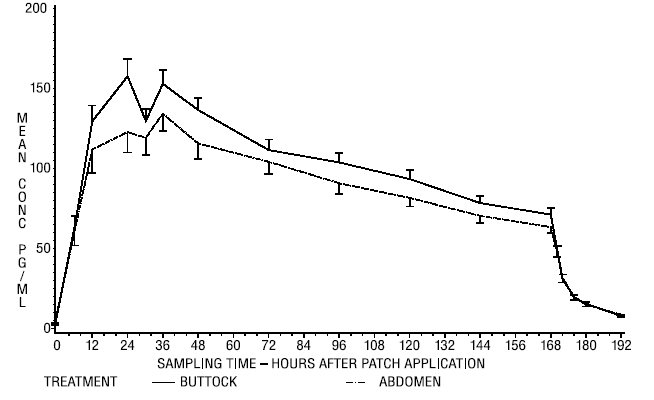
In a single dose, randomized, crossover study conducted to compare the effect of site of application, 38 postmenopausal women wore a single Climara 25 cm² transdermal system for 1 week on the abdomen and buttocks. The estradiol serum concentration profiles are shown in Figure 2. Values of Cmax and Cavg were, respectively, 25 percent and 17 percent higher with the buttock application than with the abdomen application.
Figure 2. Observed Mean (± SE) Estradiol Serum Concentrations for a One Week Application of the Climara Transdermal System (25 cm²) to the Abdomen and Buttocks of 38 Postmenopausal Women:
Table 2 provides a summary of estradiol pharmacokinetic parameters determined during evaluation of the Climara transdermal system.
Table 2. Pharmacokinetic Summary (Mean Estradiol Values):
| Climara Delivery Rate | Surface Area (cm²) | Application Site | No. of Subjects | Dosing | Cmax (pg/mL) | Cmin (pg/mL) | Cavg (pg/mL) |
|---|---|---|---|---|---|---|---|
| 0.025 | 6.5 | Abdomen | 24 | Single | 32 | 17 | 22 |
| 0.05 | 12.5 | Abdomen | 102 | Single | 71 | 29 | 41 |
| 0.1 | 25 | Abdomen | 139 | Single | 147 | 60 | 87 |
| 0.1 | 25 | Buttock | 38 | Single | 174 | 71 | 106 |
The relative standard deviation of each pharmacokinetic parameter after application to the abdomen averaged 50 percent, which is indicative of the considerable intersubject variability associated with transdermal drug delivery. The relative standard deviation of each pharmacokinetic parameter after application to the buttock was lower than that after application to the abdomen (for example, for Cmax 39 percent versus 62 percent, and for Cavg 35 percent versus 48 percent).
Distribution
The distribution of exogenous estrogens is similar to that of endogenous estrogens. Estrogens are widely distributed in the body and are generally found in higher concentrations in the sex hormone target organs. Estrogens circulate in the blood largely bound to SHBG and albumin.
Metabolism
Exogenous estrogens are metabolized in the same manner as endogenous estrogens. Circulating estrogens exist in a dynamic equilibrium of metabolic interconversions. These transformations take place mainly in the liver. Estradiol is converted reversibly to estrone, and both can be converted to estriol, which is a major urinary metabolite. Estrogens also undergo enterohepatic recirculation via sulfate and glucuronide conjugation in the liver, biliary secretion of conjugates into the intestine, and hydrolysis in the intestine followed by reabsorption. In postmenopausal women, a significant proportion of the circulating estrogens exist as sulfate conjugates, especially estrone sulfate, which serves as a circulating reservoir for the formation of more active estrogens.
Excretion
Estradiol, estrone, and estriol are excreted in the urine along with glucuronide and sulfate conjugates.
Adhesion
An open-label study of adhesion potentials of placebo transdermal systems that correspond to the 6.5 cm² and 12.5 cm² sizes of Climara was conducted in 112 healthy women of 45 to 75 years of age. Each woman applied both transdermal systems weekly, on the upper outer abdomen, for 3 consecutive weeks. It should be noted that lower abdomen and upper quadrant of the buttock are the approved sites of application for Climara.
The adhesion assessment was done visually on Days 2, 4, 5, 6, 7 of each week of transdermal system wear. A total of 1,654 adhesion observations were conducted for 333 transdermal systems of each size.
Of these observations, approximately 90 percent showed essentially no lift for both the 6.5 cm² and 12.5 cm² transdermal systems. Of the total number of transdermal systems applied, approximately 5 percent showed complete detachment for each size. Adhesion potentials of the 18.75 cm² and 25 cm² sizes of transdermal systems (0.075 mg per day and 0.1 mg per day) have not been studied.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term continuous administration of natural and synthetic estrogens in certain animal species increases the frequency of carcinomas of the breast, uterus, cervix, vagina, testis, and liver.
14. Clinical Studies
14.1 Effects on Vasomotor Symptoms
A study of 214 women 25 to 74 years of age met the qualification criteria and were randomly assigned to one of the three treatment groups: 72 to the 0.05 mg estradiol patch, 70 to the 0.1 mg estradiol patch, and 72 to placebo. Potential subjects were postmenopausal women in good general health who experienced vasomotor symptoms. Natural menopause patients had not menstruated for at least 12 months and surgical menopause patients had undergone bilateral oophorectomy at least 4 weeks before evaluation for study entry. In order to enter the 11-week treatment phase of the study, potential subjects must have experienced a minimum of five moderate to severe hot flushes per week, or a minimum of 15 hot flushes of any severity per week, for 2 consecutive weeks. Women wore the patches in a cyclical fashion (three weeks on and one week off).
During treatment, all subjects used diaries to record the number and severity of hot flushes. Subjects were monitored by clinic visits at the end of weeks 1, 3, 7, and 11 and by telephone at the end of weeks 4, 5, 8, and 9.
Adequate data for the analysis of efficacy was available from 191 subjects. The results are presented as the mean ± SD number of flushes in each of the 3 treatment weeks of each 4-week cycle. In the 0.05 mg estradiol group, the mean weekly hot flush rate across all treatment cycles decreased from 46 ± 6.5 at baseline to 20 ± 3 (-67 percent). The 0.1 mg estradiol group had a decline in the mean weekly hot flush rate from 52 ± 4.4 at baseline to 16 ± 2.4 (-72 percent). In the placebo group, the mean weekly hot flush rate declined from 53 ± 4.5 at baseline to 46 ± 6.5 (-18.1 percent). Compared with placebo, the 0.05 mg and 0.1 mg estradiol groups showed a statistically significantly larger mean decrease in hot flushes across all treatment cycles (P<0.05). When the response to treatment was analyzed for each of the three cycles of therapy, similar statistically significant differences were observed between both estradiol treatment groups and the placebo group during all treatment cycles.
In a double-blind, placebo-controlled, randomized study of 187 women receiving Climara 0.025 mg per day or placebo continuously for up to three 28-day cycles, the Climara 0.025 mg per day dosage was shown to be statistically better than placebo at weeks 4 and 12 for relief of both the frequency and severity of moderate to severe vasomotor symptoms.
Table 3. Mean Change from Baseline in the Number of Moderate to Severe Vasomotor Symptoms Intent to Treat (ITT):
| Treatment Group | Statistics | Week 4 | Week 8 | Week 12 |
|---|---|---|---|---|
| E2 Transdermal System | N | 82 | 84 | 68 |
| Mean | -6.45 | -7.69 | -7.56 | |
| SD | 4.65 | 4.76 | 4.64 | |
| Placebo | N | 83 | 71 | 65 |
| Mean | -5.11 | -5.98 | -5.98 | |
| SD | 7.43 | 8.63 | 9.69 | |
| p-value | <0.002 | <0.003 |
A second active-control trial of 193 randomized subjects was supportive of the placebo-controlled trial.
14.2 Effects on Bone Mineral Density
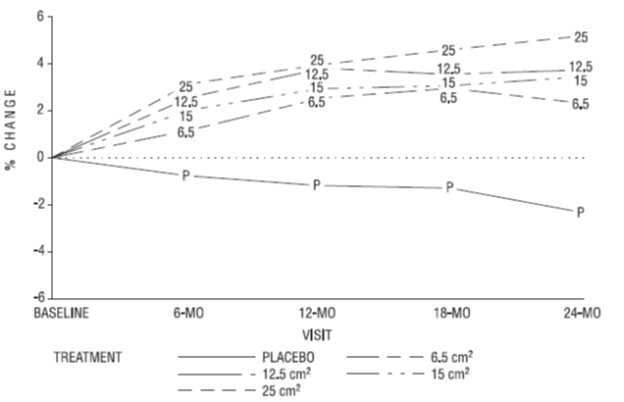
A two-year clinical trial enrolled a total of 175 healthy, hysterectomized, postmenopausal, non-osteoporotic (that is, lumbar spine bone mineral density >0.9 gm/cm²) women at 10 study centers in the United States. A total of 129 subjects were allocated to receive active treatment with 4 different doses of estradiol patches (6.5, 12.5, 15, 25 cm²) and 46 subjects were allocated to receive placebo patches. Seventy-seven percent of the randomized subjects (100 on active drug and 34 on placebo) contributed data to the analysis of percent change of anterior-posterior (A-P) spine BMD, the primary efficacy variable (see Figure 3). A statistically significant overall treatment effect at each timepoint was noted, implying bone preservation for all active treatment groups at all timepoints, as opposed to bone loss for placebo at all timepoints.
Figure 3. Mean Percent Change from Baseline in Lumbar Spine (A-P View) Bone Mineral Density By Treatment and Time Last Observation Carried Forward:
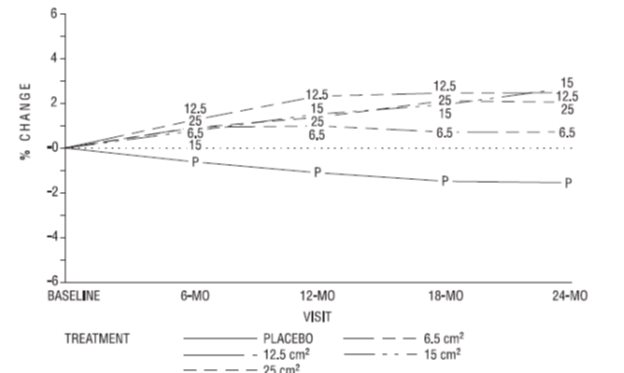
Percent change in BMD of the total hip (see Figure 4) was also statistically significantly different from placebo for all active treatment groups. This figure is based on 74 percent of the randomized subjects (95 on active drug and 34 on placebo).
Figure 4. Mean Percent Chang e from Bas eline in Total Hip by Treatment and Time Last Obs ervation Carried Forward:
14.3 Women’s Health Initiative Studies
The WHI enrolled approximately 27,000 predominantly healthy postmenopausal women in two substudies to assess the risks and benefits of daily oral CE (0.625 mg)-alone or in combination with MPA (2.5 mg) compared to placebo in the prevention of certain chronic diseases. The primary endpoint was the incidence of CHD (defined as nonfatal MI, silent MI and CHD death), with invasive breast cancer as the primary adverse outcome. A “global index” included the earliest occurrence of CHD, invasive breast cancer, stroke, PE, endometrial cancer (only in the CE plus MPA substudy), colorectal cancer, hip fracture, or death due to other causes. These substudies did not evaluate the effects of CE-alone or CE plus MPA on menopausal symptoms.
WHI Estrogen-Alone Substudy
The WHI estrogen-alone substudy was stopped early because an increased risk of stroke was observed, and it was deemed that no further information would be obtained regarding the risk and benefits of estrogen-alone in predetermined primary endpoints.
Results of the estrogen-alone substudy, which included 10,739 women (average 63 years of age, range 50 to 79: 75.3 percent White, 15.1 percent Black, 6.1 percent Hispanic, 3.6 percent Other) after an average follow-up of 7.1 years, are presented in Table 4.
Table 4. Relative and Absolute Risk Seen in the Estrogen-Alone Substudy of WHI*:
| Event | Relative Risk CE vs. Placebo (95% nCI†) | CE n=5,310 | Placebo n=5,429 |
|---|---|---|---|
| Absolute Risk per 10,000 Women-years | |||
| CHD events | 0.95 (0.78-1.16) | 54 | 57 |
| Non-fatal MIc | 0.91 (0.73-1.14) | 40 | 43 |
| CHD death‡ | 1.01 (0.71-1.43) | 16 | 16 |
| All strokes‡ | 1.33 (1.05-1.68) | 45 | 33 |
| Ischemic stroke‡ | 1.55 (1.19-2.01) | 38 | 25 |
| Deep vein thrombosis‡,§ | 1.47 (1.06-2.06) | 23 | 15 |
| Pulmonary embolism‡ | 1.37 (0.9-2.07) | 14 | 10 |
| Invasive breast cancer‡ | 0.80 (0.62-1.04) | 28 | 34 |
| Colorectal cancer‡ | 1.08 (0.75-1.55) | 17 | 16 |
| Hip fracture‡ | 0.65 (0.45-0.94) | 12 | 19 |
| Vertebral fractures‡,§ | 0.64 (0.44-0.93) | 11 | 18 |
| Lower arm/wrist fractures‡,§ | 0.58 (0.47-0.72) | 35 | 59 |
| Total fractures‡,§ | 0.71 (0.64-0.80) | 144 | 197 |
| Death due to causes¶,# | 1.08 (0.88-1.32) | 53 | 50 |
| Overall mortality‡,§ | 1.04 (0.88-1.22) | 79 | 75 |
| Global IndexÞ | 1.02 (0.92-1.13) | 206 | 201 |
* Adapted from numerous WHI publications. WHI publications can be viewed at www.nhlbi.nih.gov/whi
† Nominal confidence intervals unadjusted for multiple looks and multiple comparisons.
‡ Results are based on centrally adjudicated data for an average follow-up of 7.1 years.
§ Not included in “global index”.
¶ Results are based on an average follow-up of 6.8 years.
# All deaths, except from breast or colorectal cancer, definite or probable CHD, PE or cerebrovascular disease.
Þ A subset of the events was combined in a “global index”, defined as the earliest occurrence of CHD events, invasive breast cancer, stroke, pulmonary embolism, endometrial cancer, colorectal cancer, hip fracture, or death due to other causes.
For those outcomes included in the WHI “global index” that reached statistical significance, the absolute excess risks per 10,000 women-years in the group treated with CE-alone was 12 more strokes, while the absolute risk reduction per 10,000 women-years was 7 fewer hip fractures.9 The absolute excess risk of events included in the “global index” was a non-significant 5 events per 10,000 women-years. There was no difference between the groups in terms of all-cause mortality.
No overall difference for primary CHD events (nonfatal MI, silent MI and CHD death) and invasive breast cancer incidence in women receiving CE-alone compared with placebo was reported in final centrally adjudicated results from the estrogen-alone substudy, after an average follow-up of 7.1 years. See Table 4.
Centrally adjudicated results for stroke events from the estrogen-alone substudy, after an average follow-up of 7.1 years, reported no significant difference in the distribution of stroke subtype and severity, including fatal strokes, in women receiving estrogen-alone compared to placebo. Estrogen-alone increased the risk of ischemic stroke, and this excess risk was present in all subgroups of women examined.10 See Table 4.
Timing of initiation of estrogen-alone therapy relative to the start of menopause may affect the overall risk-benefit profile. The WHI estrogen-alone substudy stratified by age showed in women 50 to 59 years of age a non-significant trend toward reduced risk for CHD [hazard ratio (HR) 0.63 (95 percent CI, 0.36-1.09)] and overall mortality [HR 0.71 (95 percent CI, 0.46-1.11)].
WHI Estrogen Plus Progestin Substudy
The WHI estrogen plus progestin substudy was stopped early. According to the predefined stopping rule, after an average follow-up of 5.6 years of treatment, the increased risk of invasive breast cancer and cardiovascular events exceeded the specified benefits included in the “global index”. The absolute excess risk of events included in the “global index” was 19 per 10,000 women-years.
For those outcomes included in the WHI “global index” that reached statistical significance after 5.6 years of follow-up, the absolute excess risks per 10,000 women-years in the group treated with CE plus MPA were 7 more CHD events, 8 more strokes, 10 more PEs, and 8 more invasive breast cancers, while the absolute risk reduction per 10,000 women-years were 6 fewer colorectal cancers and 5 fewer hip fractures.
Results of the CE plus MPA substudy, which included 16,608 women (average 63 years of age, range 50 to 79; 83.9 percent White, 6.5 percent Black, 5.4 percent Hispanic, 3.9 percent Other), are presented in Table 5. These results reflect centrally adjudicated data after an average follow-up of 5.6 years.
Table 5. Relative and Absolute Risk Seen in the Estrogen Plus Progestin Substudy of WHI at an Average of 5.6 Years:
| Event | Relative Risk CE/MPA vs. placebo (95% nCI*) | CE/MPA n=8,506 | Placebo n=8,102 |
|---|---|---|---|
| Absolute Risk per 10,000 Women-years | |||
| CHD events | 1.23 (0.99-1.53) | 41 | 34 |
| Non-fatal MI | 1.28 (1.00-1.63) | 31 | 25 |
| CHD death | 1.10 (0.70-1.75) | 8 | 8 |
| All strokes | 1.31 (1.03-1.68) | 33 | 25 |
| Ischemic stroke | 1.44 (1.09-1.90) | 26 | 18 |
| Deep vein thrombosis† | 1.95 (1.43-2.67) | 26 | 13 |
| Pulmonary embolism | 2.13 (1.45-3.11) | 18 | 8 |
| Invasive breast cancer‡ | 1.24 (1.01-1.54) | 41 | 33 |
| Colorectal cancer | 0.61 (0.42-0.87) | 10 | 16 |
| Endometrial cancer† | 0.81 (0.48-1.36) | 6 | 7 |
| Cervical cancer† | 1.44 (0.47-4.42) | 2 | 1 |
| Hip fracture | 0.67 (0.47-0.96) | 11 | 16 |
| Vertebral fractures† | 0.65 (0.46-0.92) | 11 | 17 |
| Lower arm/wrist fractures† | 0.71 (0.59-0.85) | 44 | 62 |
| Total fractures† | 0.76 (0.69-0.83) | 152 | 199 |
| Overall mortality§ | 1.00 (0.83-1.19) | 52 | 52 |
| Global Index¶ | 1.13 (1.02-1.25) | 184 | 165 |
Adapted from numerous WHI publications. WHI publications can be viewed at www.nhlbi.nih.gov/whi. Results are based on centrally adjudicated data.
* Nominal confidence intervals unadjusted for multiple looks and multiple comparisons.
† Not included in “global index”.
‡ Includes metastatic and non-metastatic breast cancer, with the exception of in situ breast cancer.
§ All deaths, except from breast or colorectal cancer, definite or probable CHD, PE or cerebrovascular disease.
¶ A subset of the events was combined in a “global index”, defined as the earliest occurrence of CHD events, invasive breast cancer, stroke, pulmonary embolism, endometrial cancer, colorectal cancer, hip fracture, or death due to other causes
Timing of initiation of estrogen plus progestin therapy relative to the start of menopause may affect the overall risk benefit profile. The WHI estrogen plus progestin substudy stratified by age showed in women 50 to 59 years of age a non-significant trend toward reduced risk for overall mortality [HR 0.69 (95 percent CI, 0.44-1.07)].
14.4 Women’s Health Initiative Memory Study
The WHIMS estrogen-alone ancillary study of WHI enrolled 2,947 predominantly healthy hysterectomized postmenopausal women 65 to 79 years of age (45 percent were 65 to 69 years of age; 36 percent were 70 to 74 years of age; 19 percent were 75 years of age and older) to evaluate the effects of daily CE (0.625 mg)-alone on the incidence of probable dementia (primary outcome) compared to placebo.
After an average follow-up of 5.2 years, the relative risk of probable dementia for CE-alone versus placebo was 1.49 (95 percent CI, 0.83-2.66). The absolute risk of probable dementia for CE-alone versus placebo was 37 versus 25 cases per 10,000 women-years. Probable dementia as defined in the study included Alzheimer’s disease (AD), vascular dementia (VaD) and mixed types (having features of both AD and VaD). The most common classification of probable dementia in the treatment group and the placebo group was AD. Since the ancillary study was conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women [see Warnings and Precautions (5.3), and Use in Specific Populations(8.5)].
The WHIMS estrogen plus progestin ancillary study enrolled 4,532 predominantly healthy postmenopausal women 65 years of age and older (47 percent were 65 to 69 years of age; 35 percent were 70 to 74 years of age; and 18 percent were 75 years of age and older) to evaluate the effects of daily CE (0.625 mg) plus MPA (2.5 mg) on the incidence of probable dementia (primary outcome) compared to placebo.
After an average follow-up of 4 years, the relative risk of probable dementia for CE plus MPA versus placebo was 2.05 (95 percent CI, 1.21-3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 cases per 10,000 women-years. Probable dementia as defined in the study included AD, VaD and mixed types (having features of both AD and VaD). The most common classification of probable dementia in the treatment group and the placebo group was AD. Since the ancillary study was conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women [see Warnings and Precautions (5.3), and Use in Specific Populations (8.5)].
When data from the two populations were pooled as planned in the WHIMS protocol, the reported overall relative risk for probable dementia was 1.76 (95 percent CI, 1.19-2.60). Differences between groups became apparent in the first year of treatment. It is unknown whether these findings apply to younger postmenopausal women [see Warnings and Precautions (5.3), and Use in Specific Populations (8.5)].
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.