CLONAZEPAM THAME Oral solution Ref.[7175] Active ingredients: Clonazepam
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2018 Publisher: Syri Limited t/a Thame Laboratories, Unit 4, Bradfield Road, Ruislip, Middlesex, HA4 0NU, UK
Therapeutic indications
All clinical forms of epileptic disease and seizures in adults, especially absence seizures (petit mal) including atypical absence; primary or secondarily generalised tonic-clonic (grand mal), tonic or clonic seizures; partial (focal) seizures with elementary or complex symptomatology; various forms of myoclonic seizures, myoclonus and associated abnormal movements.
Posology and method of administration
Posology
The 0.5mg/5ml oral solution facilitates the administration of lower daily doses in the initial stages of treatment.
The 2mg/5ml solution should be used for maintenance and maximum dosage regimens.
Adults
Initial dosage should not exceed 1mg/day. The maintenance dosage for adults normally falls within the range 4 to 8 mg.
Elderly
The elderly are particularly sensitive to the effects of centrally depressant drugs and may experience confusion. It is recommended that the initial dosage of clonazepam should not exceed 0.5mg/day.
These are total daily dosages which should be divided into 4 doses taken at intervals throughout the day. If necessary, larger doses may be given at the discretion of the physician, up to a maximum of 20mg daily. The maintenance dose should be attained after 2 to 4 weeks of treatment.
Children
Due to the presence of ethanol in the formulation, this product is not indicated for paediatric use.
Method of administration
Treatment should be started with low doses. The dose may be increased progressively until the maintenance dose suited to the individual patient has been found.
The dosage of clonazepam must be adjusted to the needs of each individual and depends on the individual response to therapy. The maintenance dosage must be determined according to clinical response and tolerance.
The daily dose should be divided into 4 equal doses. Once the maintenance dose level has been reached, the daily amount may be given in a single dose in the evening.
Simultaneous administration of more than one antiepileptic drug is a common practice in the treatment of epilepsy and may be undertaken with clonazepam. The dosage of each drug may be required to be adjusted to obtain the optimum effect. If status epilepticus occurs in a patient receiving oral clonazepam, administration of an intravenous clonazepam injection may still control the status. Before adding clonazepam to an existing anticonvulsant regimen, it should be considered that the use of multiple anticonvulsants may result in an increase of undesired effects.
For oral use only.
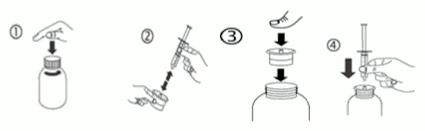
Instructions for the use of syringe:
a) Open the bottle: press the cap and turn it anticlockwise (figure 1).
b) Separate the adaptor from the syringe (figure 2). Insert the adaptor into the bottle neck (figure 3). Ensure it is properly fixed. Take the syringe and put it in the adaptor opening (figure 4).
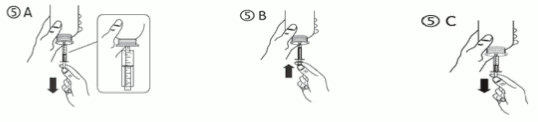
c) Turn the bottle upside down. Fill the syringe with a small amount of solution by pulling the piston down (figure 5A), then push the piston upwards in order to remove any possible bubble (figure 5B). Pull the piston down to the graduation mark corresponding to the quantity in millilitres (ml) prescribed by your doctor (figure 5C).
d) Turn the bottle the right way up (figure 6A). Remove the syringe from the adaptor (figure 6B).
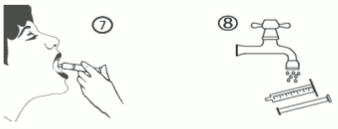
e) Empty the contents of the syringe into the patient’s mouth by pushing the piston to the bottom of the syringe (figure 7). Close the bottle with the plastic screw cap. Wash the syringe with water (figure 8).
Overdose
Symptoms
The symptoms of overdosage or intoxication vary greatly from person to person depending on age, bodyweight and individual response. Benzodiazepines commonly cause drowsiness, ataxia, dysarthria and nystagmus. Overdose of clonazepam is seldom life-threatening if the drug is taken alone, but may lead to coma, areflexia, apnoea, hypotension and cardio-respiratory depression. Coma, if it occurs, usually lasts a few hours but it may be more protracted and cyclical, particularly in elderly patients. Benzodiazepine respiratory depressant effects are more serious in patients with severe chronic obstructive airways disease.
Benzodiazepines potentiate the effects of other central nervous system depressants, including alcohol.
Management
- Maintain a clear airway and adequate ventilation if indicated.
- Supportive measures as indicated by the patient’s clinical state. In particular, patients may require symptomatic treatment for cardio-respiratory effects or central nervous system effects.
- Further absorption should be prevented using an appropriate method e.g. treatment within 1-2 hours with activated charcoal. If activated charcoal is used airway protection is imperative for drowsy patients.
- In case of mixed ingestion gastric lavage may be considered, however not as a routine measure.
- Patients who are asymptomatic at 4 hours are unlikely to develop symptoms.
- Flumazenil (Anexate), a benzodiazepine antagonist is available but should rarely be required. If CNS depression is severe consider the use of flumazenil. This should only be administered under closely monitored conditions. It has a short half-life (about an hour), therefore patients administered flumazenil will require monitoring after its effects have worn off. Flumazenil is to be used with extreme caution in the presence of drugs that reduce seizure threshold (e.g. tricyclic antidepressants). Refer to the prescribing information for flumazenil (Anexate), for further information on the correct use of this drug. Flumazenil is NOT TO BE USED IN MIXED OVERDOSE OR AS A “DIAGNOSTIC TEST”.
Warning
The use of flumazenil is not indicated in patients with epilepsy who have been treated with benzodiazepines. Although flumazenil exerts a slight intrinsic anticonvulsant effect, its abrupt suppression of the protective effect of a benzodiazepine agonist can give rise to convulsions in epileptic patients.
If excitation occurs, barbiturates should not be used.
Shelf life
Shelf life: 24 months.
Discard 30 days after first opening.
Special precautions for storage
This medicinal product does not require any special temperature storage conditions.
Keep the bottle tightly closed.
Keep the bottle in the outer carton in order to protect from light.
Nature and contents of container
Bottle: Ph. Eur. Type III Amber glass bottle.
Closure: a tamper-evident, child-resistant plastic cap with polypropylene inner, polyethylene outer and expanded polyethylene (EPE) liner.
Dosing Device: a 10ml oral syringe with 0.25ml graduation mark and a syringe adaptor.
Pack size: 150ml.
Special precautions for disposal and other handling
Any unused product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.