COMIRNATY Concentrate for dispersion for injection Ref.[10433] Active ingredients: Tozinameran
Source: European Medicines Agency (EU) Revision Year: 2021 Publisher: BioNTech Manufacturing GmbH, An der Goldgrube 12, 55131 Mainz, Germany Phone: +49 6131 9084-0 Fax: +49 6131 9084-2121 service@biontech.de
4.1. Therapeutic indications
Comirnaty 30 micrograms/dose concentrate for dispersion for injection is indicated for active immunisation to prevent COVID-19 caused by SARS-CoV-2 virus, in individuals 12 years of age and older.
The use of this vaccine should be in accordance with official recommendations.
4.2. Posology and method of administration
Posology
Individuals 12 years of age and older
Comirnaty is administered intramuscularly after dilution as a primary course of 2 doses (0.3 mL each). It is recommended to administer the second dose 3 weeks after the first dose (see sections 4.4 and 5.1).
A booster dose (third dose) of Comirnaty may be administered intramuscularly at least 6 months after the second dose in individuals 18 years of age and older. The decision when and for whom to implement a third dose of Comirnaty should be made based on available vaccine effectiveness data, taking into account limited safety data (see sections 4.4 and 5.1).
The interchangeability of Comirnaty with COVID-19 vaccines from other manufacturers to complete the primary vaccination course or the booster dose (third dose) has not been established. Individuals who have received 1 dose of Comirnaty should receive a second dose of Comirnaty to complete the primary vaccination course and for any additional doses. Doses of Comirnaty 30 micrograms/dose concentrate for dispersion for injection after dilution and Comirnaty 30 micrograms/dose dispersion for injection are considered interchangeable.
Severely immunocompromised aged 12 years and older
A third dose may be given at least 28 days after the second dose to individuals who are severely immunocompromised (see section 4.4).
Paediatric population
There is a paediatric formulation available for children 5 to 11 years of age (i.e. 5 to less than 12 years of age). For details, please refer to the Summary of Product Characteristics for Comirnaty 10 micrograms/dose concentrate for dispersion for injection.
Elderly population
No dosage adjustment is required in elderly individuals ≥ 65 years of age. The safety and immunogenicity of a booster dose (third dose) of Comirnaty in individuals 65 years of age and older is based on safety and immunogenicity data in adults 18 to 55 years of age.
Method of administration
Comirnaty 30 micrograms/dose concentrate for dispersion for injection should be administered intramuscularly after dilution (see section 6.6).
After dilution, vials of Comirnaty contain 6 doses of 0.3 mL of vaccine. In order to extract 6 doses from a single vial, low dead-volume syringes and/or needles should be used. The low dead-volume syringe and needle combination should have a dead volume of no more than 35 microlitres. If standard syringes and needles are used, there may not be sufficient volume to extract a sixth dose from a single vial. Irrespective of the type of syringe and needle:
- Each dose must contain 0.3 mL of vaccine.
- If the amount of vaccine remaining in the vial cannot provide a full dose of 0.3 mL, discard the vial and any excess volume.
- Do not pool excess vaccine from multiple vials.
The preferred site is the deltoid muscle of the upper arm.
Do not inject the vaccine intravascularly, subcutaneously or intradermally.
The vaccine should not be mixed in the same syringe with any other vaccines or medicinal products.
For precautions to be taken before administering the vaccine, see section 4.4.
For instructions regarding thawing, handling and disposal of the vaccine, see section 6.6.
4.9. Overdose
Overdose data is available from 52 study participants included in the clinical trial that due to an error in dilution received 58 micrograms of Comirnaty. The vaccine recipients did not report an increase in reactogenicity or adverse reactions.
In the event of overdose, monitoring of vital functions and possible symptomatic treatment is recommended.
6.3. Shelf life
Unopened vial
Frozen vial
9 months when stored at -90°C to -60°C.
Within the 9-month shelf life unopened vials may be stored and transported at -25°C to -15°C for a single period of up to 2 weeks and can be returned to -90°C to -60°C.
When stored frozen at -90°C to -60°C, 195-vial packs of the vaccine can be thawed at 2°C to 8°C for 3 hours or individual vials can be thawed at room temperature (up to 30°C) for 30 minutes.
Thawed vial
1 month at 2°C to 8°C within the 9-month shelf life.
Within the 1-month shelf life at 2°C to 8°C, up to 12 hours may be used for transportation.
Prior to use, the unopened vial can be stored for up to 2 hours at temperatures up to 30°C.
Thawed vials can be handled in room light conditions.
Once thawed, the vaccine should not be re-frozen.
Handling of temperature excursions once removed from the freezer
Stability data indicate that the unopened vial is stable for up to:
- 24 hours when stored at temperatures from -3°C to 2°C
- a total of 4 hours when stored at temperatures from 8°C to 30°C; this includes the 2 hours at up to 30°C detailed above
This information is intended to guide healthcare professionals only in case of temporary temperature excursion.
Transfers of frozen vials stored at ultra-low temperature (< -60°C)
- Closed-lid vial trays containing 195 vials removed from ultra-low temperature frozen storage (< -60°C) may be at temperatures up to 25°C for up to 5 minutes.
- Open-lid vial trays, or vial trays containing less than 195 vials, removed from ultra-low temperature frozen storage (< -60°C) may be at temperatures up to 25°C for up to 3 minutes.
- After vial trays are returned to frozen storage following temperature exposure up to 25°C, they must remain in frozen storage for at least 2 hours before they can be removed again.
Transfers of frozen vials stored at -25°C to -15°C
- Closed-lid vial trays containing 195 vials removed from frozen storage (-25°C to -15°C) may be at temperatures up to 25°C for up to 3 minutes.
- Open-lid vial trays, or vial trays containing less than 195 vials, removed from frozen storage (-25°C to -15°C) may be at temperatures up to 25°C for up to 1 minute.
Once a vial is removed from the vial tray, it should be thawed for use.
Diluted medicinal product
Chemical and physical in-use stability, including during transportation, has been demonstrated for 6 hours at 2ºC to 30ºC after dilution in sodium chloride 9 mg/mL (0.9%) solution for injection. From a microbiological point of view, unless the method of dilution precludes the risk of microbial contamination, the product should be used immediately. If not used immediately, in-use storage times and conditions are the responsibility of the user.
6.4. Special precautions for storage
Store in a freezer at -90°C to -60°C.
Store in the original package in order to protect from light.
During storage, minimise exposure to room light, and avoid exposure to direct sunlight and ultraviolet light.
For storage conditions after thawing and dilution of the medicinal product, see section 6.3.
6.5. Nature and contents of container
0.45 mL concentrate in a 2 mL clear multidose vial (type I glass) with a stopper (synthetic bromobutyl rubber) and a purple flip-off plastic cap with aluminium seal. Each vial contains 6 doses, see section 6.6.
Pack size: 195 vials.
6.6. Special precautions for disposal and other handling
Handling instructions
Comirnaty should be prepared by a healthcare professional using aseptic technique to ensure the sterility of the prepared dispersion.
Dose verification of comirnaty 30 micrograms/dose concentrate for dispersion for injection (12 years and older):
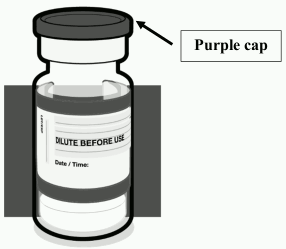 | • Verify that the vial has a purple plastic cap. • If the vial has a grey plastic cap, please make reference to the Summary of Product Characteristics for Comirnaty 30 micrograms/dose dispersion for injection. • If the vial has an orange plastic cap, please make reference to the Summary of Product Characteristics for Comirnaty 10 micrograms/dose concentrate for dispersion for injection. |
Thawing prior to dilution of Comirnaty 30 micrograms/dose concentrate for dispersion for injection (12 years and older):
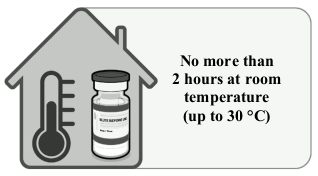 | • The multidose vial is stored frozen and must be thawed prior to dilution. Frozen vials should be transferred to an environment of 2°C to 8°C to thaw; a 195 vial pack may take 3 hours to thaw. Alternatively, frozen vials may also be thawed for 30 minutes at temperatures up to 30°C for immediate use. • The unopened vial can be stored for up to 1 month at 2°C to 8°C within the 9-month shelf life. Within the 1-month shelf life at 2°C to 8°C, up to 12 hours may be used for transportation. • Allow the thawed vial to come to room temperature. Prior to use, the unopened vial can be stored for up to 2 hours at temperatures up to 30°C. Thawed vials can be handled in room light conditions. • Gently invert the vial 10 times prior to dilution. Do not shake. • Prior to dilution, the thawed dispersion may contain white to off-white opaque amorphous particles. |
Dilution of Comirnaty 30 micrograms/dose concentrate for dispersion for injection (12 years and older):
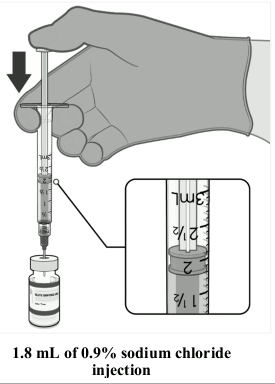 | • The thawed vaccine must be diluted in its original vial with 1.8 mL sodium chloride 9 mg/mL (0.9%) solution for injection, using a 21 gauge or narrower needle and aseptic techniques. |
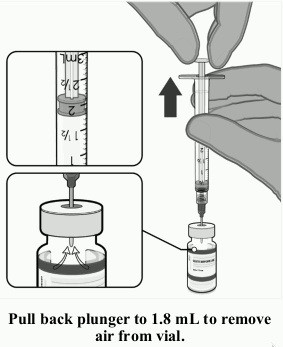 | • Equalise vial pressure before removing the needle from the vial stopper by withdrawing 1.8 mL air into the empty diluent syringe. |
 | • Gently invert the diluted dispersion 10 times. Do not shake. • The diluted vaccine should present as an off-white dispersion with no particulates visible. Do not use the diluted vaccine if particulates or discolouration are present. |
 | • The diluted vials should be marked with the appropriate date and time. • After dilution, store at 2ºC to 30ºC and use within 6 hours, including any transportation time. • Do not freeze or shake the diluted dispersion. If refrigerated, allow the diluted dispersion to come to room temperature prior to use. |
Preparation of individual 0.3 mL doses of Comirnaty 30 micrograms/dose concentrate for dispersion for injection (12 years and older):
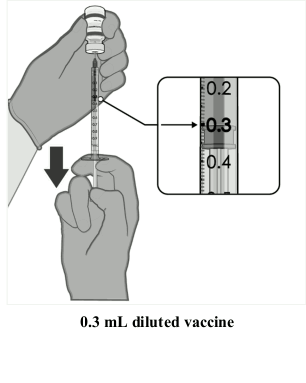 | • After dilution, the vial contains 2.25 mL from which 6 doses of 0.3 mL can be extracted. • Using aseptic technique, cleanse the vial stopper with a single-use antiseptic swab. • Withdraw 0.3 mL of Comirnaty. Low dead-volume syringes and/or needles should be used in order to extract 6 doses from a single vial. The low dead-volume syringe and needle combination should have a dead volume of no more than 35 microlitres. If standard syringes and needles are used, there may not be sufficient volume to extract a sixth dose from a single vial. • Each dose must contain 0.3 mL of vaccine. • If the amount of vaccine remaining in the vial cannot provide a full dose of 0.3 mL, discard the vial and any excess volume. • Discard any unused vaccine within 6 hours after dilution. |
Disposal
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.