CORLOPAM Solution for injection Ref.[10053] Active ingredients: Fenoldopam
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
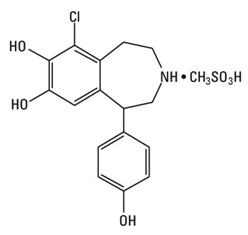
CORLOPAM (Fenoldopam Mesylate Injection, USP) is a dopamine D1-like receptor agonist. The product is formulated as a solution to be diluted for intravenous infusion. Chemically it is 6-chloro-2,3,4,5-tetrahydro-1-(4-hydroxyphenyl)-[1H]-3-benzazepine-7,8-diol methanesulfonate with the following structure:
fenoldopam mesylate
Fenoldopam mesylate is a white to off-white powder with a molecular weight of 401.87 and a molecular formula of C16H16ClNO3•CH3SO3H. It is sparingly soluble in water, ethanol and methanol, and is soluble in propylene glycol.
Each 1 mL contains, in sterile aqueous solution, citric acid 3.44 mg; fenoldopam mesylate equivalent to fenoldopam 10 mg; propylene glycol 518 mg; sodium citrate dihydrate 0.61 mg; sodium metabisulfite 1 mg.
| Dosage Forms and Strengths |
|---|
|
10 mg/mL solution in single-dose vial 10 mg/mL solution in single-dose ampule |
| How Supplied | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
Drugs
| Drug | Countries | |
|---|---|---|
| CORLOPAM | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.