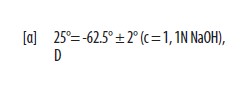CUPRIMINE Capsule Ref.[10789] Active ingredients: Penicillamine
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
Penicillamine, USP is a chelating agent used in the treatment of Wilson’s disease. It is also used to reduce cystine excretion in cystinuria and to treat patients with severe, active rheumatoid arthritis unresponsive to conventional therapy (see INDICATIONS). It is 3-mercapto-D-valine. It is a white, or practically white, crystalline powder, freely soluble in water, slightly soluble in alcohol, and insoluble in ether, acetone, benzene, and carbon tetrachloride. Although its configuration is D, it is levorotatory as usually measured:
calculated on a dried basis.
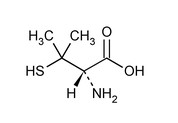
The empirical formula is C5H11NO2S, giving it a molecular weight of 149.21.
The structural formula is:
It reacts readily with formaldehyde or acetone to form a thiazolidine-carboxylic acid. CUPRIMINE (penicillamine) Capsules, USP for oral administration contain 250 mg of penicillamine. Each capsule contains the following inactive ingredients: D&C Yellow No. 10, gelatin, lactose monohydrate, magnesium stearate, and titanium dioxide.
| How Supplied |
|---|
|
CUPRIMINE Capsules, USP 250 mg, are pale yellow to ivory opaque hard gelatin capsules imprinted with “ATON 705” on the cap and “CUPRIMINE” on the body. They are supplied as follows: NDC 25010-705-15 in bottles of 100 Distributed by: Bausch Health US, LLC, Bridgewater, NJ 08807 USA Manufactured by: Suven Pharmaceuticals Limited, Telangana, India 502307 |
Drugs
| Drug | Countries | |
|---|---|---|
| CUPRIMINE | Brazil, Canada, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.