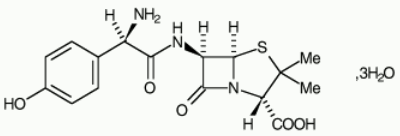
CURAM Powder for oral suspension / Film-coated tablet Ref.[50585] Active ingredients: Amoxicillin Clavulanic acid
Source: Health Products Regulatory Authority (ZA) Revision Year: 2022 Publisher: Novartis New Zealand Limited, PO Box 99102, Newmarket, Auckland 1149 Telephone: 0800 354 335
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: J01CR02 – Combinations of penicillins, including beta-lactamase inhibitors.
Antibiotic class: Semi-synthetic penicillin compounded with a beta-lactamase inhibitor.
Amoxicillin trihydrate:
Potassium clavulanate:
Mechanism of action
Curam film-coated tablets and oral suspension are antibiotic preparations compounded for oral administration from amoxicillin trihydrate – a beta-lactam antibacterial penicillin and the potassium salt of clavulanic acid – a beta-lactamase inhibitor. Amoxicillin/clavulanic acid has a notably broad spectrum of activity against bacterial pathogens of clinical importance to general practice and secondary/tertiary care.
Antibiotic nature and mode of action
Amoxicillin is an aminopenicillin type broad-spectrum antibiotic that possesses activity against many Gram-positive and Gram-negative microorganisms. Amoxicillin is a bactericidal, cellwall active agent that inhibits bacterial cell wall synthesis by binding to penicillin binding proteins and inhibits the cross-linking of bacterial peptidoglycan. However, amoxicillin is susceptible to degradation by beta-lactamases so it’s intrinsic spectrum of activity excludes bacteria producing these enzymes.
Clavulanic acid is a beta-lactam, structurally related to the penicillins. It possesses the ability to inactivate a wide range of beta-lactamase enzymes commonly found in microorganisms resistant to penicillins and cephalosporins. In particular, it has good activity against the clinically important plasmid mediated beta-lactamases frequently responsible for transferred drug resistance. It is generally less effective against chromosomally-mediated type 1 betalactamases.
When appropriately combined with amoxicillin, clavulanic acid inhibits the degradation of amoxicillin by beta-lactamase enzymes and effectively extends the antibacterial spectrum of amoxicillin to include many bacteria normally resistant to amoxicillin, other penicillins and cephalosporins.
Susceptibility data
The prevalence of resistance may vary geographically and temporally for selected species. Local resistance information is desirable, particularly when treating severe infections. The following information only provides approximate guidance on the probabilities that microorganisms will be susceptible to amoxicillin/clavulanic acid.
Susceptible
Susceptible Gram-positive aerobes include Bacillus anthracis 1, Corynebacterium spp., Enterococcus faecalis 1, Listeria monocytogenes, Nocardia asteroides, Staphylococcus aureus 1, Coagulase negative staphylococci 1 (including Staphylococcus epidermidis 1), Streptococcus spp.; Streptococcus pneumoniae; Group A streptococci (including Streptococcus pyogenes); Group B streptococci (including Streptococcus agalactiae); Viridans group streptococci.
Susceptible Gram-positive anaerobes include Clostridium spp., Peptococcus spp., Peptostreptococcus spp.
Susceptible Gram-negative aerobes include Bordetella pertussis, Brucella spp., Gardnerella vaginalis, Haemophilus influenzae 1, Helicobacter pylori, Legionella species, Moraxella catarrhalis 1, Neisseria gonorrhoeae 1, Neisseria meningitidis 1, Pasteurella multocida, Proteus mirabilis 1, Proteus vulgaris 1, Vibrio cholerae, Yersinia enterocolitica 1.
Susceptible Gram-negative anaerobes include: Bacteroides spp. 1 (including Bacteroides fragilis), Fusobacterium spp. 1
Other susceptible pathogens include Borrelia burgdorferi, Chlamydiae spp., Leptospira icterohaemorrhagiae, Treponema pallidum.
Intermediate
Partially susceptible Gram-positive aerobes include Enterococcus faecium 1.
Partially susceptible Gram-negative aerobes include Escherichia coli 1; Klebsiella spp. 1; Klebsiella pneumoniae 1; Klebsiella oxytoca 1; Salmonella spp. 1; Salmonella hadar 1; Salmonella typhimurium 1; Shigella spp. 1.
Notes:
1 Some members of these species of bacteria produce beta-lactamase, rendering them insensitive to amoxicillin alone.
Resistance
Although, amoxicillin/clavulanic acid may exhibit in vitro activity against methicillin/oxacillin resistant Staphylococcus aureus (MRSA) and coagulase-negative staphylococci (MRS) it is not clinically effective and isolates should therefore be considered resistant. Rare betalactamase negative, ampicillin resistant (BLNAR) strains of H. influenzae should also be considered resistant to amoxicillin/clavulanic acid despite the apparent in vitro susceptibility of some BLNAR strains.
Resistant Gram-positive aerobes include Staphylococcus aureus (MRSA) and Coagulasenegative staphylococci (MRS).
Resistant Gram-negative aerobes include Acinetobacter spp.; Citrobacter spp.; Enterobacter spp.; Haemophilus influenzae (BLNAR); Morganella morganii; Providencia spp.; Pseudomonas aeruginosa; Serratia spp.; Stenotrophomonas maltophilia.
Other resistant pathogens include Mycoplasma spp. and Rickettsia spp.
Clinically relevant MIC ranges
According to the US National Committee on Clinical Laboratory Standards (NCCLS) in 2001, the following breakpoints have been defined for amoxicillin/clavulanic acid: Enterobacteriaceae: NMT 8/4 mcg/mL susceptible, 16/8 mcg/mL intermediate, NLT 32/16 mcg/mL resistant; Staphylococcus spp. and Haemophilus spp.: NMT 4/2 mcg/mL susceptible, NLT 8/4 mcg/mL resistant; Streptococcus pneumoniae: NMT 2/1 mcg/mL susceptible, 4/2 mcg/mL intermediate, NLT 8/4 mcg/mL resistant; Anaerobic bacteria: NMT 4/2 mcg/mL susceptible, 8/4 mcg/mL intermediate, NLT 16/8 mcg/mL resistant.
No NCCLS breakpoint is stipulated for Vibrio cholerae, however, ampicillin susceptibility (NMT 8 mcg/mL susceptible, 16 mcg/mL intermediate, NLT 32 mcg/mL resistant) is representative for amoxicillin/clavulanic acid.
For Enterococcus spp. penicillin and ampicillin susceptibility (NMT 8 mcg/mL susceptible, NLT 16 mcg/mL resistant) may be used to predict the susceptibility to amoxicillin/clavulanic acid.
Clinical trials
No data available.
5.2. Pharmacokinetic properties
Absorption
Amoxicillin and clavulanic acid are fully dissociated in aqueous solution at physiological pH. Both components are rapidly and well absorbed by the oral route of administration. Absorption of amoxicillin/clavulanic acid is optimised when taken at the start of a meal.
Two separate studies measured the mean pharmacokinetic parameters in fasting healthy volunteers for amoxicillin/clavulanic acid 625 mg tablets equivalent to amoxicillin 500 mg and clavulanic acid 125 mg against the two components given separately. Following a single oral dose equivalent to amoxicillin 500 mg: amoxicillin/clavulanic acid 625 mg gave a plasma concentration-time profile characterised by a peak corresponding to Cmax of 6.5 mg/L at tmax of 1.5 hours with AUC of 23.2 mg.h/L and elimination half life of 1.3 hours; amoxicillin 500 mg alone gave a plasma concentration-time profile characterised by a peak corresponding to Cmax of 6.5 mg/L at tmax of 1.3 hours with AUC of 19.5 mg.h/L and elimination half life of 1.1 hours. Similarly, following a single oral dose equivalent to clavulanic acid 125 mg: amoxicillin/clavulanic acid 625 mg gave a plasma concentration-time profile characterised by a peak corresponding to Cmax of 2.8 mg/L at tmax of 1.3 hours with AUC of 7.3 mg.h/L and elimination half life of 0.8 hours; clavulanic acid 125 mg alone gave a plasma concentrationtime profile characterised by a peak corresponding to Cmax of 3.4 mg/L at tmax of 0.9 hours with AUC of 7.8 mg.h/L and elimination half life of 0.7 hours. The studies suggest that amoxicillin serum concentrations achieved with amoxicillin/clavulanic acid are similar to those produced by the oral administration of equivalent doses of amoxicillin alone.
Distribution
Intravenous administration of the prescribed dose of amoxicillin/clavulanic acid provides therapeutic levels of both constituents in the tissues and interstitial fluids including gall bladder, skin, abdominal, adipose and muscle tissues, synovial and peritoneal fluids, bile and pus. Neither amoxicillin nor clavulanic acid is highly protein bound; studies show that about 13% to 25% of total plasma drug content of each compound is bound to protein. From animal studies, there is no evidence to suggest that either compound accumulates in any organ.
Amoxicillin, like most penicillins, can be detected in breast milk. Trace quantities of clavulanate can also be detected in breast milk. With the exception of the risk of sensitisation associated with this excretion, there are no known detrimental effects for the breastfed infant.
Reproduction studies in animals have shown that both amoxicillin and clavulanic acid penetrate the placental barrier. However, no evidence of impaired fertility or harm to the foetus was detected.
Metabolism
Amoxicillin is partly metabolised with between 10 to 25% of the initial dose found in the urine as the inactive penicilloic acid. Clavulanic acid is extensively metabolised. The inactive metabolites 2,5-dihydro-4-(2-hydroxyethyl)-5-oxo-1H-pyrrole-3-carboxylic acid and 1-amino-4-hydroxy-butan-2-one are eliminated by urinary and biliary excretion while the terminal metabolite, carbon dioxide, is eliminated in expired air and water.
Excretion
As with other penicillins, the major route of elimination for amoxicillin is via the kidney, whereas for clavulanate it is by both renal and non-renal mechanisms. Approximately 60 to 70% of the amoxicillin content and approximately 40 to 65% of the clavulanic acid content are excreted unchanged in urine during the first 6 hours after administration of a single 500/125 mg tablet.
5.3. Preclinical safety data
Genotoxicity
No data available.
Carcinogenicity
No data available.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.