CUTIVATE Lotion Ref.[11123] Active ingredients: Fluticasone
Source: FDA, National Drug Code (US) Revision Year: 2018
Product description
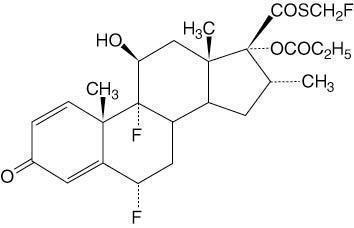
CUTIVATE (fluticasone propionate) Lotion USP, 0.05% contains fluticasone propionate, USP [S-Fluoromethyl 6α, 9α-difluoro-11β-hydroxy-16α-methyl-3-oxo-17α-propionyloxyandrosta-1,4-diene-17β-carbothioate], a synthetic fluorinated corticosteroid, for topical use. The topical corticosteroids constitute a class of primarily synthetic steroids used as anti-inflammatory and antipruritic agents.
Chemically, fluticasone propionate, USP is C25H31F3O5S.
It has the following structural formula:
Fluticasone propionate, USP has a molecular weight of 500.6. It is a white to off-white powder and is practically insoluble in water, freely soluble in dimethyl sulfoxide and dimethylformamide, and slightly soluble in methanol and 95% ethanol.
Each gram of CUTIVATE Lotion USP contains 0.5 mg fluticasone propionate, USP in a white to off white lotion base of cetomacrogol 1000, cetostearyl alcohol, citric acid monohydrate, dimethicone 350, imidurea, isopropyl myristate, methylparaben, propylene glycol, propylparaben, purified water, and sodium citrate.
| Dosage Forms and Strengths |
|---|
|
Lotion, 0.05%. Each gram of CUTIVATE Lotion USP contains 0.5 mg fluticasone propionate, USP in a white to off-white lotion base. CUTIVATE Lotion USP is supplied in 120 mL bottles. |
| How Supplied |
|---|
|
CUTIVATE (fluticasone propionate) Lotion USP, 0.05% is white to off-white in color, and supplied as follows: 120 mL bottle NDC 10337-434-04 |
Drugs
| Drug | Countries | |
|---|---|---|
| CUTIVATE | Cyprus, Ecuador, Estonia, Hong Kong, Lithuania, Malta, Mexico, Netherlands, Poland, Romania, Singapore, Turkey, United Kingdom, United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.