CUVPOSA Oral solution Ref.[10794] Active ingredients: Glycopyrronium
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
Glycopyrrolate is a competitive inhibitor of acetylcholine receptors that are located on certain peripheral tissues, including salivary glands. Glycopyrrolate indirectly reduces the rate of salivation by preventing the stimulation of these receptors.
12.2. Pharmacodynamics
Glycopyrrolate inhibits the action of acetylcholine on salivary glands thereby reducing the extent of salivation.
12.3. Pharmacokinetics
Absorption
In a parallel study of children (n=6 per group) aged 7-14 years undergoing intraocular surgery, subjects received either intravenous (IV) or oral glycopyrrolate as a premedication. The mean absolute bioavailability of oral glycopyrrolate tablets was low (approximately 3%) and highly variable among subjects (range 1.3 to 13.3%). A similar pattern of low and variable relative bioavailability is seen in adults.
Analysis of population pharmacokinetic data from normal adults and children with cerebral palsy associated chronic moderate to severe drooling failed to demonstrate linear pharmacokinetics across the dose range. In the same analysis, population estimates of the apparent oral clearance (scaled by weight in children and adults) ranged from 5.28-38.95 L/hr/kg for healthy adults and 8.07-25.65 L/hr/kg for patients with cerebral palsy, a reflection of the low and highly variable oral bioavailability of glycopyrrolate.
Absorption of CUVPOSA (fasting) was compared to that of a marketed glycopyrrolate oral tablet. The Cmax after oral solution administration was 23% lower compared to tablet administration and AUC0-inf was 28% lower after oral solution administration. Mean Cmax after oral solution administration in the fasting state was 0.318 ng/mL, and mean AUC0-24 was 1.74 ng∙hr/mL. Mean time to maximum plasma concentration for CUVPOSA was 3.1 hours, and mean plasma half-life was 3.0 hours.
In healthy adults, a high fat meal was shown to significantly affect the absorption of glycopyrrolate oral solution (10 mL, 1 mg/5 mL). The mean Cmax under fed high fat meal conditions was approximately 74% lower than the Cmax observed under fasting conditions. Similarly, mean AUC0-T was reduced by about 78% by the high fat meal compared with the fasting AUC0-T. A high fat meal markedly reduces the oral bioavailability of CUVPOSA. Therefore, CUVPOSA should be dosed at least one hour before or two hours after meals. Pharmacokinetic results (mean ± SD) are described in Table 3.
Table 3. Pharmacokinetic Parameters (mean±SD) for CUVPOSA, Fasting and Fed, in Healthy Adults:
| Cmax (ng/mL) | Tmax (hrs) | AUC0-T (ng∙hr/mL) | AUC0-Inf (ng∙hr/mL) | T1/2 (hrs) | |
|---|---|---|---|---|---|
| Fasting (n=37) | 0.318 ± 0.190 | 3.10 ± 1.08 | 1.74 ± 1.07 | 1.81 ± 1.09 | 3.0 ± 1.2 |
| Fed (n=36) | 0.084 ± 0.081 | 2.60 ± 1.12 | 0.38 ± 0.14 | 0.46 ± 0.13* | 3.2 ± 1.1* |
* n=35
Distribution
After IV administration, glycopyrrolate has a mean volume of distribution in children aged 1 to 14 years of approximately 1.3 to 1.8 L/kg, with a range from 0.7 to 3.9 L/kg. In adults aged 60-75 years, the volume of distribution was lower (0.42 L/kg +/- 0.22).
Metabolism
In adult patients who underwent surgery for cholelithiasis and were given a single IV dose of tritiated glycopyrrolate, approximately 85% of total radioactivity was excreted in urine and <5% was present in T-tube drainage of bile. In both urine and bile, >80% of the radioactivity corresponded to unchanged drug. These data suggest a small proportion of IV glycopyrrolate is excreted as one or more metabolites.
Elimination
Approximately 65-80% of an IV glycopyrrolate dose was eliminated unchanged in urine in adults. In two studies, after IV administration to pediatric patients ages 1-14 years, mean clearance values ranged from 1.01-1.41 L/kg/hr (range 0.32-2.22 L/kg/hr). In adults, IV clearance values were 0.54 ± 0.14 L/kg/hr.
Pediatrics
The estimated apparent clearance of glycopyrrolate from a population pharmacokinetic analysis (scaled by weight in children and adults) of oral and IV data was found to be 13.2 L/hr/kg or 92.7 L/hr for a typical 70 kg subject. In the same population based analysis, gender was not identified as having an effect on either glycopyrrolate clearance or systemic exposure.
Gender
Population pharmacokinetic evaluation of adults and children administered IV or oral glycopyrrolate identified no effect of gender on glycopyrrolate clearance or systemic exposure.
Race
The pharmacokinetics of glycopyrrolate by race has not been characterized.
Elderly
Glycopyrrolate pharmacokinetics have not been characterized in the elderly.
Renal Impairment
In one study, glycopyrrolate 4 mcg/kg was administered intravenously in uremic patients undergoing renal transplantation surgery. Mean AUC (10.6 mcg∙h/L), mean plasma clearance (0.43 L/hr/kg) and mean 3-hour urinary excretion (0.7%) for glycopyrrolate were significantly different than those of control patients (3.73 µg∙h/L, 1.14 L/hr/kg, and 50%, respectively). These results suggest that elimination of glycopyrrolate is severely impaired in patients with renal failure.
Hepatic Impairment
Glycopyrrolate is largely renally eliminated. The pharmacokinetics of glycopyrrolate have not been evaluated in patients with hepatic impairment.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
When glycopyrrolate was administered via oral gavage to mice for up to 24 months at dosages of 2.5, 7, and 20 mg/kg/day in both genders, resulting in systemic exposures (estimated AUC0-inf values) approximately 0.1, 0.3, and 0.8 times, respectively, the estimated systemic exposure in humans at the MRHD (9 mg per day, administered in three divided doses), no significant changes in tumor incidence were observed when compared to control.
When glycopyrrolate was administered via oral gavage to rats for up to 24 months at dosages of 5, 15, and 40 mg/kg/day in both genders, resulting in systemic exposures approximately 0.2, 0.8, and 2 times, respectively, the estimated systemic exposure in humans at the MRHD, no significant changes in tumor incidence were observed when compared to control.
Glycopyrrolate did not elicit any genotoxic effects in the Ames mutagenicity assay, the human lymphocyte chromosome aberration assay, or the micronucleus assay.
Glycopyrrolate was assessed for effects on fertility or general reproductive function in rats. Rats of both genders received glycopyrrolate at dosages up to 100 mg/kg/day via oral gavage, resulting in systemic exposures (estimated AUC0-inf values) in males and females up to approximately 11 and 15 times, respectively, the estimated systemic exposure in humans at the MRHD. No treatment-related effects on fertility or reproductive parameters were observed in either gender in this study.
14. Clinical Studies
CUVPOSA was evaluated in a multi-center, randomized, double-blind, placebo-controlled, parallel, eight-week study for the control of pathologic drooling in children (Study 1). The study enrolled 38 subjects aged 3-23 years; thirty-six subjects were aged 3-16 years and two patients were greater than 16 years. The subjects were male or female, weighed at least 13 kg (27 lbs), and had cerebral palsy, mental retardation, or another neurologic condition associated with problem drooling defined as drooling in the absence of treatment so that clothing became damp on most days (approximately five to seven days per week). Subjects were randomized in a 1:1 fashion to receive CUVPOSA or placebo. Doses of study medication were titrated over a 4-week period to optimal response beginning at 0.02 mg/kg three times a day increasing doses in increments of approximately 0.02 mg/kg three times per day every 5-7 days, not to exceed the lesser of approximately 0.1 mg/kg three times per day or 3 mg three times per day.
Subjects were evaluated on the 9-point modified Teacher's Drooling Scale (mTDS), which is presented below. The mTDS evaluations were recorded by parents/caregivers 3 times daily approximately two hours post-dose on evaluation days during pre-treatment baseline and at Weeks 2, 4, 6 and 8 of therapy.
Modified Teacher's Drooling Scale:
1 = Dry: never drools
2 = Mild: only the lips are wet; occasionally
3 = Mild: only the lips are wet; frequently
4 = Moderate: wet on the lips and chin; occasionally
5 = Moderate: wet on the lips and chin; frequently
6 = Severe: drools to the extent that clothing becomes damp; occasionally
7 = Severe: drools to the extent that clothing becomes damp; frequently
8 = Profuse: clothing, hands, tray, and objects become wet; occasionally
9 = Profuse: clothing, hands, tray, and objects become wet; frequently
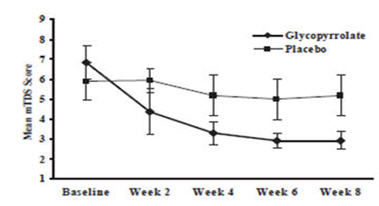
Responders were defined as subjects with at least a 3-point reduction in mean daily mTDS scores from baseline to Week 8. Table 4 presents the proportion of responders at Week 8 and Figure 1 presents the mean mTDS values from baseline through Week 8.
Table 4. Percentage of Responders at Week 8:
| CUVPOSA Group (N=20) | Placebo Group (N=18) |
|---|---|
| 15/20 (75%) | 2/18 (11%) |
Figure 1. Mean (± 2 Standard Errors) mDTS Scores:
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.