CYSTADROPS Eye drops solution Ref.[7690] Active ingredients: Mercaptamine
Source: European Medicines Agency (EU) Revision Year: 2021 Publisher: Recordati Rare Diseases, Immeuble Le Wilson, 70, Avenue du Général de Gaulle, 92800 Puteaux, France
Therapeutic indications
Cystadrops is indicated for the treatment of corneal cystine crystal deposits in adults and children from 2 years of age with cystinosis.
Posology and method of administration
Treatment with Cystadrops should be initiated under the supervision of a physician experienced in the management of cystinosis.
Posology
The recommended dose is one drop in each eye, 4 times a day during waking hours. The recommended interval between each instillation is 4 hours. The dose could be decreased progressively (to a minimum total daily dose of 1 drop in each eye) depending on the results of ophthalmic examination (such as, corneal cystine crystal deposits, photophobia).
If the patient misses an instillation, the patient should be told to continue the treatment with the next instillation.
The dose should not exceed 4 drops a day in each eye.
The accumulation of corneal cystine crystals increases if Cystadrops is discontinued. The treatment should not be stopped.
Paediatric population
Cystadrops may be used in paediatric patients from 2 years of age at the same dose as in adults (see section 5.1).
The safety and efficacy of Cystadrops in children aged less than 2 years has not been established. No data are available.
Method of administration
For ocular use.
Before the first admnistration, in order to facilitate the administration, the patient should be told to bring back Cystadrops at room temperature. After first opening, the patient should be told to keep the dropper bottle at room temperature.
To avoid sticky eyes in the morning, the patient should be advised to apply the last drop of the day at least 30 minutes before going to bed.
To prevent contamination of the dropper tip and solution, care must be taken not to touch the eyelids, surrounding areas, or other surfaces with the dropper tip of the dropper bottle.
The patient should be told to discard the dropper bottle after 7 days of use.
In case of concomitant therapy with other topical ocular medicinal products, an interval of ten minutes should be allowed between successive applications. Eye ointments should be administered last.
Overdose
Overdose is unlikely to occur with ocular administration.
In case of accidental ingestion, monitoring and symptomatic management of the patient should be implemented.
Shelf life
6 months.
After first opening: 7 days. Store below 25°C. Do not refrigerate. Keep the dropper bottle tightly closed in the outer carton in order to protect from light.
Special precautions for storage
Before first opening:
Store in a refrigerator (2°C-8°C). Keep the vial in the outer carton in order to protect from light.
For storage conditions after first opening of the medicinal product, see section 6.3.
Nature and contents of container
5 mL solution in a 10 mL amber glass vial closed by a bromobutyl stopper and sealed with an aluminium tear-off cap. A PVC dropper applicator with HDPE closure is packed separately and included in each carton box.
Each carton box contains 1 vial and 1 dropper applicator.
Pack of 1 carton box or multipack containing 4 carton boxes.
Not all pack sizes may be marketed.
Special precautions for disposal and other handling
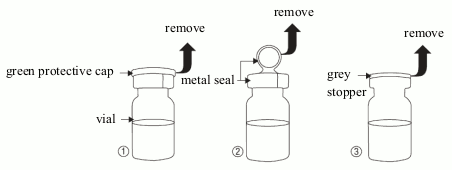
The patient should be advised to follow the instructions below for opening of the vial and attachement of the dropper applicator:
- Wash your hands carefully in order to avoid microbiological contamination of the content in the vial.
- Remove the green protective cap (picture 1).
- Remove the metal seal (picture 2).
- Remove the grey stopper (picture 3) from the vial.
- Do not touch the opening of the vial after removing the grey stopper.
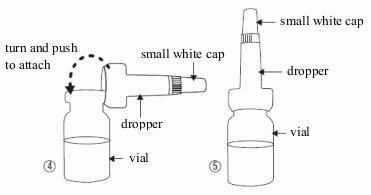
- Take the dropper out of its sachet, without touching the end intended to be attached to the vial, attach it (picture 4) to the vial and do not remove it.
- Make sure that you do not lose the small white cap (picture 5) that comes on the top of the dropper.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.