DATSCAN Solution for injection Ref.[10327] Active ingredients: Ioflupane ¹²³I
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
DaTscan [Ioflupane I 123 Injection] is a sterile, pyrogen-free radiopharmaceutical for intravenous injection. The clear and colorless solution is supplied in single-use vials in which each milliliter contains 0.07 to 0.13 µg ioflupane, 74 MBq (2 mCi) of iodine 123 (as ioflupane I 123) at calibration time, 5.7 mg acetic acid, 7.8 mg sodium acetate and 0.05 mL (5%) ethanol. The pH of the solution is between 4.2 and 5.2.
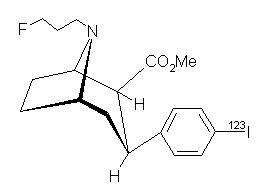
Ioflupane I 123 has the following structural formula:
11.1 Physical Characteristics
Iodine 123 is a cyclotron-produced radionuclide that decays to 123Te by electron capture and has a physical half-life of 13.2 hours. The photon that is useful for detection and imaging studies is listed in Table 2.
Table 2. Principal Radiation Emission Data – Iodine-123:
| Radiation | Energy Level (keV) | Abundance (%) |
|---|---|---|
| Gamma | 159 | 83 |
11.2 External Radiation
The specific gamma-ray constant for iodine 123 is 1.6 R/mCi-hr at 1 cm. The first half-value thickness of lead (Pb) for iodine 123 is 0.04 cm. The relative transmission of radiation emitted by the radionuclide that results from interposition of various thicknesses of Pb is shown in Table 3 (e.g., the use of 2.16 cm Pb will decrease the external radiation exposure by a factor of about 1,000).
Table 3. Reduction in In-air Collision Kerma Caused by Lead Shielding*:
| Shield Thickness cm of lead (Pb) | Reduction in In-air Collision Kerma |
|---|---|
| 0.04 | 0.5 |
| 0.13 | 10-1 |
| 0.77 | 10-2 |
| 2.16 | 10-3 |
| 3.67 | 10-4 |
* Calculation based on attenuation and energy-transfer coefficients obtained from National Institute of Standards & Technology Internal Report NISTIR 5632.
| Dosage Forms and Strengths |
|---|
|
Single-use vials containing 185 MBq (5 mCi) in 2.5 mL sterile solution for intravenous injection [74 MBq (2 mCi) per mL at calibration time]. |
| How Supplied |
|---|
|
DaTscan is supplied in 10-mL glass vials containing a total volume of 2.5 mL of solution with a total radioactivity of 185 MBq (5 mCi) at calibration time. Each vial is enclosed in a lead container of appropriate thickness. NDC 17156-210-01 |
Drugs
| Drug | Countries | |
|---|---|---|
| DATSCAN | Austria, Cyprus, Estonia, Spain, France, Hong Kong, Croatia, Ireland, Japan, Lithuania, Poland, Romania, Singapore, Turkey, United Kingdom, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.