DAURISMO Film-coated tablet Ref.[10664] Active ingredients: Glasdegib
Source: European Medicines Agency (EU) Revision Year: 2021 Publisher: Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050 Bruxelles, Belgium
4.3. Contraindications
Hypersensitivity to the active substance or to any of the excipients listed in section 6.1.
4.4. Special warnings and precautions for use
Embryo-foetal toxicity
Based on its mechanism of action and findings from animal embryo-foetal developmental toxicity studies, Daurismo can cause embryo-foetal death or severe birth defects when administered to a pregnant woman. Pregnant women should be advised of the potential risk to the foetus (see section 4.6).
Daurismo should not be used during pregnancy and in women of childbearing potential not using contraception. The pregnancy status of female patients of childbearing potential should be verified prior to initiating treatment with Daurismo. Women of childbearing potential should be advised to always use effective contraception during treatment with Daurismo and for at least 30 days after the last dose (see section 4.6).
Males
Glasdegib may be present in semen. Male patients with female partners should be advised of the potential risk of exposure through semen and to always use effective contraception, including a condom (with spermicide, if available), even after vasectomy, to avoid exposure of a pregnant partner or a female partner of childbearing potential during treatment with Daurismo and for at least 30 days after the last dose (see section 4.6).
If a female patient or female partner of a male patient becomes pregnant, or suspects a pregnancy during treatment with Daurismo or during the 30 days after the last dose, they must inform their healthcare provider immediately (see section 4.6).
Based on non-clinical safety findings, glasdegib has the potential to impair reproductive function in males. Men should seek advice on effective fertility preservation prior to initiating treatment with Daurismo (see section 4.6).
QT interval prolongation
In a randomised study (Study 1) of patients with AML and high-risk MDS (myelodysplastic syndrome) treated with Daurismo with low-dose cytarabine vs. low-dose cytarabine alone, Grade ¾ ECG QT prolonged was reported in 3.5% of patients treated with Daurismo with low-dose cytarabine compared to 2.4% of the patients treated with low-dose cytarabine alone.
Electrolytes should be assessed prior to initiation of Daurismo, at least once weekly for the first month, and then once monthly for the duration of therapy. Electrolyte abnormalities should be corrected.
Concomitant medicinal products should be assessed. For medicinal products that have known QT prolonging effects and/or strong CYP3A4 inhibitor potential, alternativesshould be considered.
ECGs should be monitored prior to the initiation of Daurismo, approximately one week after initiation, and then once monthly for the next two months to assess for QTc prolongation. In patients with congenital long QT syndrome, congestive heart failure, electrolyte abnormalities, or those who are taking medicinal products with known QT prolonging effects, more frequent ECG monitoring is recommended. ECG should be repeated if abnormal. Abnormalities should be managed promptly, and dose modifications should be considered (see sections 4.2 and 4.5).
Muscle-related adverse events
In Study 1, muscle spasms were observed in 22.6% of patients treated with Daurismo with low-dose cytarabine compared to 4.8% of the patients treated with low-dose cytarabine alone.
All patients starting therapy with Daurismo must be informed of the risk of muscle-related adverse events. They must be instructed to report promptly any unexplained muscle pain, tenderness or weakness occurring during treatment with Daurismo or if symptoms persist after discontinuing treatment.
Serum CK levels should be obtained prior to initiating Daurismo and as clinically indicated thereafter (e.g., if muscle signs and symptoms are reported). Management of high-grade CK elevation based on current standards of medical practice and following appropriate treatment guidelines is recommended. Dose modification or management recommendations should be followed (see section 4.2).
Renal impairment
Patients with pre-existing renal impairment or risk factors for renal dysfunction should be monitored closely. Renal function should be assessed prior to initiation of therapy and at least once weekly for the first month of therapy with Daurismo. Electrolytes and renal function should be monitored once monthly for the duration of therapy (see section 4.2).
Excipients
Lactose intolerance
Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take this medicinal product.
Sodium content
This medicinal product contains less than 1 mmol sodium (23 mg) per tablet, that is to say essentially ‘sodium-free’.
4.5. Interaction with other medicinal products and other forms of interaction
Effects of other medicinal products on the pharmacokinetics of glasdegib
In vitro, CYP3A4 is responsible for the majority of glasdegib depletion and contributed to the formation of other minor oxidative metabolites, with CYP2C8 and UGT1A9 playing a minor role in the metabolism of glasdegib.
Substances that may increase glasdegib plasma concentration
CYP3A4 inhibitors
Ketoconazole, a strong inhibitor of CYP3A4, dosed at 400 mg once daily for 7 days, increased the mean area under the curve (AUCinf) by ~2.4-fold and maximum plasma concentration (Cmax) by 40% of a single 200 mg oral dose of glasdegib in healthy subjects. Caution should be used when administering concomitantly with strong CYP3A4 inhibitors (e.g., boceprevir, cobicistat, conivaptan, itraconazole, ketoconazole, posaconazole, telaprevir, troleandomycin, voriconazole, ritonavir, grapefruit or grapefruit juice ) as an increase in glasdegib plasma concentration may occur. If possible, alternate concomitant medicinal product with no or minimal CYP3A4 inhibition potential is recommended (see section 4.4).
Gastric pH altering medicinal products
Coadministration of a single 100 mg glasdegib dose under fasted condition with multiple doses of the proton-pump inhibitor (PPI), rabeprazole, resulted in no change in glasdegib plasma exposure (AUCinf ratio: 100.6%). Concomitant administration of glasdegib with acid-reducing agents (including PPIs, H2-receptor antagonists, and locally acting antacids) is permitted.
Substances that may decrease glasdegib plasma concentration
CYP3A4 inducers
Rifampicin, a strong inducer of CYP3A4, administered at a dose of 600 mg once daily for 11 days, reduced the mean AUCinf by 70% and Cmax by 35% of a single 100 mg dose of glasdegib in healthy subjects. Concomitant use with strong CYP3A4 inducers (e.g., rifampicin, carbamazepine, enzalutamide, mitotane, phenytoin and St. John’s Wort)should be avoided, as this is likely to decrease glasdegib plasma concentrations.
Simulations using physiologic-based pharmacokinetic modelling suggested that coadministration of efavirenz (a moderate inducer of CYP3A4) with glasdegib decreased glasdegib AUCinf by 55% and Cmax by 25%. Concomitant use of moderate CYP3A4 inducers (e.g., bosentan, efavirenz, etravirine, modafinil, nafcillin) should be avoided as they may also reduce glasdegib plasma concentrations (see section 4.4). If concomitant use of moderate CYP3A4 inducers cannot be avoided, the dose of Daurismo should be increased (see section 4.2).
Effect of glasdegib on the pharmacokinetics of other medicinal products
Pharmacodynamic interactions
Medicinal products known to prolong QT interval
Glasdegib may prolong QT interval. Therefore, the concomitant use of glasdegib with other medicinal products known to prolong QT interval or induce Torsades de Pointes should be carefully considered (see sections 4.2 and 4.4).
Pharmacokinetic interactions
Drug transporters
In vitro studies indicated that glasdegib may have the potential to inhibit P-glycoprotein (P-gp, gastrointestinal [GI] tract) and breast cancer resistance protein (BCRP, systemically and at the GI tract) mediated transport at clinically relevant concentrations. Therefore, narrow therapeutic index substrates of P-gp (e.g., digoxin) or BCRP should be used with caution in combination with glasdegib.
In vitro studies of transporter inhibition
In vitro studies indicated that glasdegib may have the potential to inhibit (MATE)1 and MATE2K at clinically relevant concentrations.
4.6. Fertility, pregnancy and lactation
Women of childbearing potential / Contraception in males and females
If Daurismo is used in women of childbearing potential, they should be advised to avoid becoming pregnant. The pregnancy status of female patients of childbearing potential should be verified prior to initiating treatment. If the patient becomes pregnant while taking Daurismo, the patient should be apprised of the potential hazard to the foetus.
Based on its mechanism of action and findings from animal embryo-foetal developmental studies, Daurismo can cause foetal harm when administered to a pregnant woman. Women of childbearing potential who are receiving this medicinal product should always use effective contraception during treatment with Daurismo and for at least 30 days after the last dose. If a female patient becomes pregnant, or suspects a pregnancy, during treatment with Daurismo or during the 30 days after the last dose, she must notify her healthcare provider immediately (see section 4.4).
Males
Glasdegib may be present in semen. Male patients should not donate semen during treatment with Daurismo and for at least 30 days after the last dose. Male patients with female partners should be advised of the potential risk of exposure through semen and to always use effective contraception, including a condom (with spermicide, if available), even after a vasectomy, to avoid exposure of a pregnant partner or a female partner of childbearing potential during treatment with Daurismo and for at least 30 days after the last dose. Male patients must inform their healthcare provider immediately if their female partner becomes pregnant during treatment with Daurismo or during the 30 days after the last dose (see section 4.4).
Pregnancy
There are no data on the use of Daurismo in pregnant women. Based on its mechanism of action and findings in animal embryo-foetal developmental toxicity studies, glasdegib can cause foetal harm when administered to a pregnant woman (see section 5.3). Daurismo should not be used during pregnancy and in women of childbearing potential not using contraception (see section 4.4).
Breast-feeding
No studies have been conducted in humans to assess the effect of glasdegib on milk production, its presence in breast milk, or its effects on the breast-fed child. It is unknown whether glasdegib and its metabolites are excreted in human milk. Given the potential for serious adverse reactions in breast-feeding children from glasdegib, breast-feeding is not recommended during treatment with Daurismo and for at least one week after the last dose (see section 5.3).
Fertility
Based on non-clinical safety findings, glasdegib has the potential to impair reproductive function in males. Men should seek advice on effective fertility preservation prior to initiating treatment with Daurismo. Based on its mechanism of action, Daurismo may impair female fertility (see section 5.3).
4.7. Effects on ability to drive and use machines
Daurismo has minor influence on the ability to drive and use machines. However, patients experiencing fatigue or other symptoms (e.g., muscle cramps, pain, nausea) affecting the ability to react normally while taking Daurismo should exercise caution when driving or operating machines.
4.8. Undesirable effects
Summary of the safety profile
The overall safety profile of Daurismo is based on data from clinical studies, including Study 1 in 84 patients with AML (N=75) and high-risk MDS (N=9). The median exposure to Daurismo across the dataset was 75.5 days.
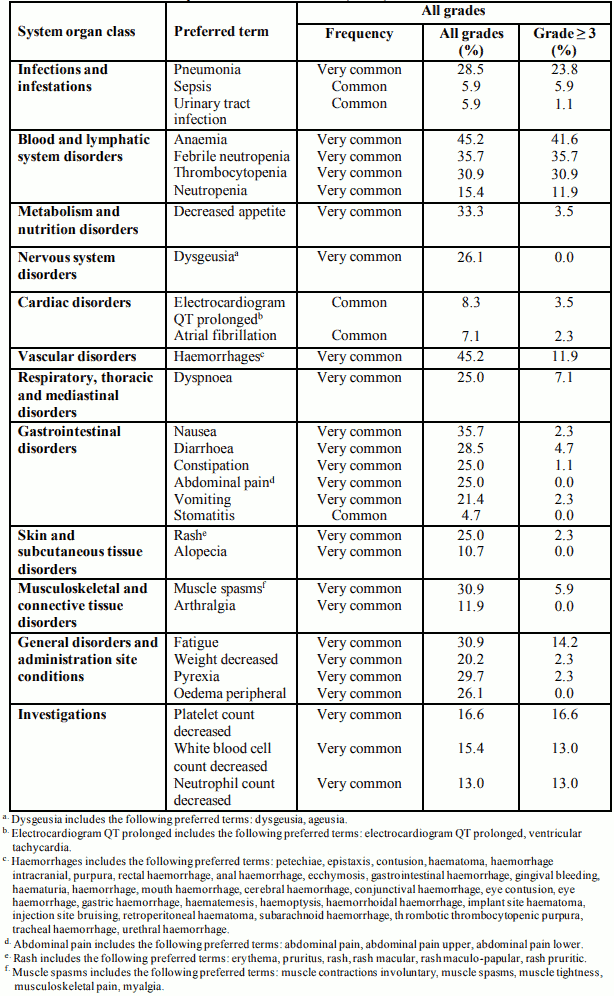
The most frequently (≥20%) reported adverse reactions in patients receiving Daurismo were anaemia (45.2%), haemorrhages (45.2%), febrile neutropenia (35.7%), nausea (35.7%), decreased appetite (33.3%), fatigue (30.9%), muscle spasms (30.9%), thrombocytopenia (30.9%), pyrexia (29.7%), diarrhoea (28.5%), pneumonia (28.5%), dysgeusia (26.1%), oedema peripheral (26.1%), constipation (25.0%), abdominal pain (25.0%), rash (25.0%), dyspnoea (25.0%) vomiting (21.4%), and weight decreased (20.2%).
The most frequently reported adverse reactions leading to dose reductions in patients receiving Daurismo were muscle spasms (4.7%), fatigue (3.5%), febrile neutropenia (3.5%), anaemia (2.3%), thrombocytopenia (2.3%), and electrocardiogram QT prolonged (2.3%). The most frequently reported adverse reactionsleading to permanent discontinuation in patients receiving Daurismo were pneumonia (5.9%), febrile neutropenia (3.5%), and nausea (2.3%).
Tabulated list of adverse reactions
Table 6 presents adverse reactions reported with Daurismo. The adverse reactions are listed by system organ class and frequency category. Frequency categories are defined as: very common (≥1/10) and common (≥1/100 to <1/10). Within each frequency grouping, adverse reactions are presented in decreasing order of all grade frequencies.
Table 6. Adverse reactions reported in clinical studies (N=84):
Description of selected adverse reactions
Muscle spasms
In Study 1, muscle spasms (all grades) were reported in 22.6% of patients in the Daurismo with low-dose cytarabine arm compared to 4.8% in the low-dose cytarabine alone arm. Grades 3 and 4 muscle spasms were reported in 4.7% of patients in the Daurismo with low-dose cytarabine arm compared to none in the low-dose cytarabine alone arm.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system listed in Appendix V.
6.2. Incompatibilities
Not applicable.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.