DAYBUE Oral solution Ref.[107361] Active ingredients: Trofinetide
Source: FDA, National Drug Code (US) Revision Year: 2023
Product description
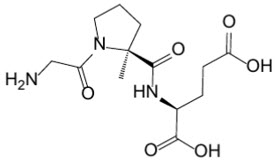
Trofinetide is designated chemically as (2S)-2-{[(2S)-1-(2-aminoacetyl)-2-methylpyrrolidine-2-carbonyl]amino}pentanedioic acid (IUPAC). Its empirical formula is C13H21N3O6 and its molecular weight is 315.33 g/mol.
The chemical structure is:
Trofinetide is a white to off-white solid and is freely soluble in water.
DAYBUE is a pink to red, oral solution with each 5 mL containing 1 g of trofinetide (200 mg/mL). The oral solution also contains FD&C Red No. 40, maltitol, methylparaben sodium, propylparaben sodium, purified water, strawberry flavor, and sucralose as inactive ingredients.
| Dosage Forms and Strengths |
|---|
|
Trofinetide oral solution: 200 mg/mL of a pink to red, strawberry flavored solution. |
| How Supplied |
|---|
|
DAYBUE (trofinetide) 200 mg/mL oral solution is a pink to red, strawberry flavored solution supplied in a nominal 500 mL round high-density polyethylene (HDPE) multi-dose bottle with a child-resistant closure containing 450 mL of oral solution (NDC 63090-660-01). Marketed by: Acadia Pharmaceuticals Inc. San Diego, CA 92130 USA |
Drugs
| Drug | Countries | |
|---|---|---|
| DAYBUE | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.