DEFITELIO Solution for injection Ref.[9989] Active ingredients: Defibrotide
Source: FDA, National Drug Code (US) Revision Year: 2016
Product description
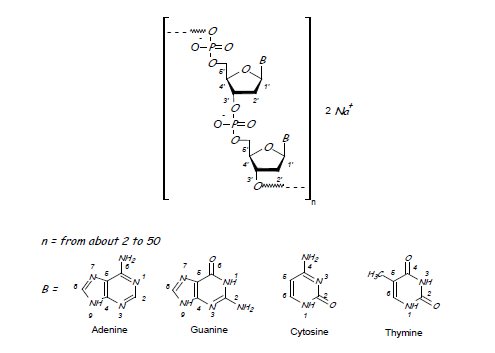
Defibrotide sodium is an oligonucleotide mixture with profibrinolytic properties. The chemical name of defibrotide sodium is polydeoxyribonucleotide, sodium salt. Defibrotide sodium is a polydisperse mixture of predominantly single-stranded (ss) polydeoxyribonucleotide sodium salts derived from porcine intestinal tissue having a mean weighted molecular weight of 13-20 kDa, and a potency of 27-39 and 28-38 biological units per mg as determined by two separate assays measuring the release of a product formed by contact between defibrotide sodium, plasmin and a plasmin substrate. The primary structure of defibrotide sodium is shown below.
DEFITELIO (defibrotide sodium) injection is a clear, light yellow to brown, sterile, preservative-free solution in a single-patient-use vial for intravenous use. Each milliliter of the injection contains 80 mg of defibrotide sodium and 10 mg of Sodium Citrate, USP, in Water for Injection, USP. Hydrochloric Acid, NF, and/or Sodium Hydroxide, NF, may have been used to adjust pH to 6.8-7.8.
| Dosage Forms and Strengths |
|---|
|
Injection: 200 mg/2.5 mL (80 mg/mL) of defibrotide sodium as a clear, light yellow to brown solution in a single-patient-use glass vial. |
| How Supplied |
|---|
|
DEFITELIO (defibrotide sodium) injection is supplied in a single-patient-use, clear glass vial as a clear, light yellow to brown, sterile, preservative-free solution for intravenous infusion. Each vial (NDC 68727-800-01) contains 200 mg/2.5 mL (at a concentration of 80 mg/mL) of defibrotide sodium. Each carton of DEFITELIO (defibrotide sodium) injection (NDC 68727-800-02) contains 10 vials. |
Drugs
| Drug | Countries | |
|---|---|---|
| DEFITELIO | Austria, Brazil, Canada, Estonia, Finland, France, Croatia, Ireland, Israel, Japan, Lithuania, Netherlands, Poland, Romania, United Kingdom, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.