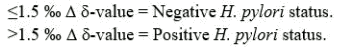DIABACT UBT Tablet Ref.[28028] Active ingredients: Urea ¹³C
Source: Health Products Regulatory Authority (IE) Revision Year: 2021 Publisher: Laboratoires Mayoly Spindler, 6 avenue de leurope BP 51, 78401 CHATOU Cedex, France
4.1. Therapeutic indications
This medicinal product is for diagnostic use only.
For In vivo diagnosis of gastroduodenal primary or remaining Helicobacter pylori infection.
4.2. Posology and method of administration
Diabact UBT tablet is for oral administration.
One tablet as a single dose at one test occasion. The patient should fast for at least six hours preceding the test. An initial breath test sample is taken after which the tablet is swallowed whole with a glass of water. A breath sample is taken after ten minutes.
It is important to follow the instructions for use, described in section 6.6.
4.9. Overdose
Overdose is unlikely to occur in the intended clinical circumstances. No case of overdose has been reported.
6.3. Shelf life
4 years.
6.4. Special precautions for storage
Do not store above 25°C. Store in the original package in order to protect from light.
6.5. Nature and contents of container
Diabact UBT pack sizes:
1 tablet in aluminium blister, 2 sampling tubes with blue stopper and 2 sampling tubes with red stopper, 1 disposable straw and extra bar code labels.
10 × 1 tablets in aluminium blister (tablets only).
Not all pack sizes may be marketed.
6.6. Special precautions for disposal and other handling
The test should be performed in the presence of a qualified person.
If the test is to be carried out in the morning, the patient should fast overnight and not eat breakfast. If the test is to be carried out later in the day, or if fasting is a problem for the patient, then a light breakfast only, e.g. tea and toast, is recommended. If the patient has eaten a heavy meal then it will be necessary to fast for six hours prior to the test.
The collection of breath samples can be performed using tubes or breath bags. Breath bags are provided separately.
Handle sampled tubes and breath bags with care and avoid any damage which may cause leakage.
Stepwise description of the test procedure using tubes
To perform the test, 4 test tubes with stoppers and 1 straw are used.
Keep one of the extra bar code labels as a reference label for the patient journal.
- The patient should begin the procedure by exhaling into the two baseline (00-MINUTES) tubes (blue stopper)
- Unscrew the stopper and place the straw in the bottom of the test tube.
- Take a deep breath and exhale gently into the tube.
- Remove the straw from the tube and close the tube with the stopper. Check that the stopper is properly closed.
- Repeat the test with the other 00-MINUTES test tube
- Swallow the tablet with a glass of water. Wait for 10 minutes in an upright position (standing or sitting position).
- Exhale into the two 10-MINUTES tubes (red stopper) in the same way as described above.
After the test has been performed, the tubes should be placed in the box and sent to the laboratory for analysis.
Stepwise description of the test procedure using breath bags
To perform the test, 1 double breath bag or 2 single breath bags and 1 mouth piece are used.
- The patient should begin the procedure by exhaling through the mouth piece for the baseline (00-MINUTE) breath bag.
- Remove the stopper cap from the breath bag hose and connect a mouth piece to the hose.
- Take a deep breath and exhale into the mouth piece which is connected to the breath bag.
- Remove the mouthpiece from the breath bag and close the breath bag with the stopper cap.
- Swallow the tablet with a glass of water. Wait for 10 minutes in an upright position (standing or sitting position)
- Exhale into the unused side of the double breath bag or to another single breath bag for the 10-MINUTES sample in the same way as described above.
Label the breath bags in order to identify the different samples (e.g.“00-MINUTE” and “10-MINUTE”).
Analysis of breath samples
One must ensure that an approved and certified laboratory performs the analysis. Laboratories and analysis equipment used should comply with EU directive: Council Directive 93/42/EEC/98/79/EC. This directive implies that the analysis equipment should be CE marked and approved for analysis of breath tests.
Explanation of analysis results
D d-value;
The difference in parts per thousand (‰) between the 00-MINUTE value and the 10 MINUTE value.
H. pylori status:
Any unused product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.