DIAFORMIN Film-coated tablet Ref.[50318] Active ingredients: Metformin
Source: Pharmaceutical Benefits Scheme (AU) Revision Year: 2022 Publisher: Alphapharm Pty Ltd trading as Viatris, Level 1, 30 The Bond, 30-34 Hickson Road, Millers Point NSW 2000, www.viatris.com.au, Phone: 1800 274 276
Product name and form
DIAFORMIN - Metformin hydrochloride.
| Pharmaceutical Form |
|---|
|
500 mg tablet: 15.65 mm x 7.25 mm, oblong, white, clear coated, tablet debossed "MF/1" on one side and blank on the other. 850 mg tablet: 12.5 mm normal convex, white, clear film-coated tablet, debossed "MF" over "2" on one side and "G" on the other. 1000 mg tablet: 19 mm x 10.5 mm, oval, film coated white tablet, debossed "MF/3" on one side and "G" on the other side. |
Qualitative and quantitative composition
The tablets contain metformin hydrochloride as the active ingredient and are available in three strengths 500 mg, 850 mg and 1000 mg.
Excipients with known effect: trace quantities of sulfites.
For the full list of excipients, see Section 6.1 LIST OF EXCIPIENTS.
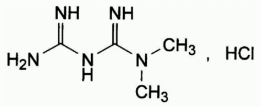
Chemical structure
Metformin hydrochloride is a white, crystalline powder which is odourless or almost odourless and hygroscopic. It is freely soluble in water, slightly soluble in ethanol (96%), and practically insoluble in chloroform and in ether.
Chemical name: 1,1-dimethylbiguanide hydrochloride
Molecular formula: C4H11N5, HCl
Molecular weight: 165.6
CAS number: 1115-70-4
| Active Ingredient |
|---|
|
Metformin is a biguanide with antihyperglycaemic effects, lowering both basal and postprandial plasma glucose. It does not stimulate insulin secretion and therefore does not produce hypoglycaemia. |
| List of Excipients |
|---|
|
The tablets contain the following excipients: povidone and magnesium stearate, and are film-coated with a proprietary coating ingredient, OPADRY complete film coating system 20C59060 Clear (ARTG PI No: 106044). |
Pack sizes and marketing
DIAFORMIN 500: Aluminium/PVC Blister packs of 10 (sample) and 100 tablets.
DIAFORMIN: PP pails packs* of 22700 tablets and 4540 tablets.
DIAFORMIN 850: Aluminium/PVC Blister packs and HDPE bottles of 60 tablets.
DIAFORMIN 1000: Aluminium/PVC Blister packs of 10, 30, 60 and 90 tablets. PP pails packs* of 11380 tablets and 2276 tablets.
* Bulk pack for dose administration to aid Packers
Some strengths, pack sizes and/or pack types may not be marketed.
Australian Register of Therapeutic Goods (ARTG)
AUST R 73806 - DIAFORMIN 500 metformin hydrochloride 500mg tablet blister pack.
AUST R 299825 - DIAFORMIN metformin hydrochloride 500 mg tablet bulk pack.
AUST R 73808 - DIAFORMIN 850 metformin hydrochloride 850mg tablet blister pack.
AUST R 73807 - DIAFORMIN 850 metformin hydrochloride 850mg tablet bottle.
AUST R 82207 - DIAFORMIN 1000 metformin hydrochloride 1000mg tablet blister pack.
AUST R 294057 - DIAFORMIN 1000 metformin 1000mg (as hydrochloride) tablet bulk pack.
Marketing authorization holder
Alphapharm Pty Ltd trading as Viatris, Level 1, 30 The Bond, 30-34 Hickson Road, Millers Point NSW 2000, www.viatris.com.au, Phone: 1800 274 276
Marketing authorization dates and numbers
05/04/2000 (DIAFORMIN)
05/04/2000 (DIAFORMIN 850)
23/08/2002 (DIAFORMIN 1000)
Drugs
| Drug | Countries | |
|---|---|---|
| DIAFORMIN | Australia, Brazil, Hong Kong, Turkey, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.