DIPROLENE AF Cream Ref.[10874] Active ingredients: Betamethasone
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
DIPROLENE AF (augmented betamethasone dipropionate) Cream 0.05% contains betamethasone dipropionate USP, a synthetic adrenocorticosteroid, for topical use in a cream base. Betamethasone, an analog of prednisolone, has a high degree of corticosteroid activity and a slight degree of mineralocorticoid activity. Betamethasone dipropionate is the 17,21-dipropionate ester of betamethasone.
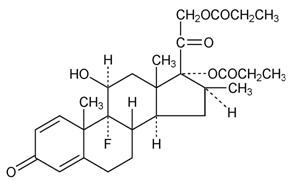
Chemically, betamethasone dipropionate is 9-fluoro-11β,17,21-trihydroxy-16β-methylpregna-1,4-diene-3,20-dione 17,21-dipropionate, with the empirical formula C28H37FO7, a molecular weight of 504.6, and the following structural formula:
Betamethasone dipropionate is a white to creamy white, odorless crystalline powder, insoluble in water.
Each gram of DIPROLENE AF Cream 0.05% contains: 0.643 mg betamethasone dipropionate USP (equivalent to 0.5 mg betamethasone) in a white cream base of carbomer 940; ceteareth-30; chlorocresol; cyclomethicone; glyceryl oleate/propylene glycol; propylene glycol; purified water; sodium hydroxide; sorbitol solution; white petrolatum; and white wax.
| Dosage Forms and Strengths |
|---|
|
Cream, 0.05%. Each gram of DIPROLENE AF Cream, 0.05% contains 0.643 mg betamethasone dipropionate (equivalent to 0.5 mg betamethasone) in a white cream base. |
| How Supplied |
|---|
|
DIPROLENE AF Cream 0.05% is a white cream supplied in 15-g (NDC 0085-0517-01) and 50-g (NDC 0085-0517-04) tubes. Distributed by: Merck Sharp & Dohme Corp., a subsidiary of MERCK & CO., INC., Whitehouse Station, NJ 08889, USA |
Drugs
| Drug | Countries | |
|---|---|---|
| DIPROLENE | Canada, France, Netherlands, New Zealand, Poland, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.