DIURIL Solution for injection Ref.[10059] Active ingredients: Chlorothiazide
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
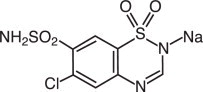
Intravenous Sodium DIURIL (chlorothiazide sodium) is a diuretic and antihypertensive. It is 6-chloro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide monosodium salt and its molecular weight is 317.71.
Its empirical formula is C7H5ClN3NaO4S2 and its structural formula is:
Intravenous Sodium DIURIL is a sterile lyophilized white powder and is supplied in a vial containing:
Chlorothiazide sodium equivalent to chlorothiazide 0.5 g
Inactive ingredients: Mannitol 0.25 g, Sodium hydroxide to adjust pH.
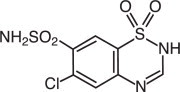
DIURIL (chlorothiazide) is a diuretic and antihypertensive. It is 6-chloro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide. Its empirical formula is C7H6ClN3O4S2 and its structural formula is:
It is a white, or practically white, crystalline powder with a molecular weight of 295.72, which is very slightly soluble in water, but readily soluble in dilute aqueous sodium hydroxide. It is soluble in urine to the extent of about 150 mg per 100 mL at pH 7.
| How Supplied |
|---|
|
Intravenous Sodium DIURIL is a dry, sterile lyophilized white powder usually in plug form, supplied in vials containing chlorothiazide sodium equivalent to 0.5 g of chlorothiazide. NDC 76478-711-40 Distributed by: Akorn, Inc., Lake Forest, IL 60045 |
Drugs
| Drug | Countries | |
|---|---|---|
| DIURIL | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.