DORYX MPC Tablet Ref.[27552] Active ingredients: Doxycycline
Source: FDA, National Drug Code (US) Revision Year: 2021
Product description
Doryx MPC (doxycycline hyclate delayed-release tablets) for oral use, contain doxycycline hyclate, a tetracycline class drug synthetically derived from oxytetracycline, in a delayed-release formulation consisting of pellets with a modified polymer enteric coat that has increased acid resistance.
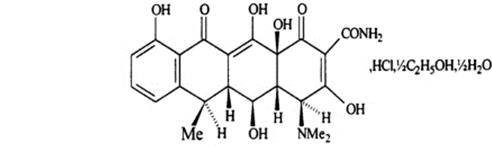
The structural formula for doxycycline hyclate is:
with a molecular formula of C22H24N2O8, HCl, ½ C2H6O, ½ H2O and a molecular weight of 512.9. The chemical name for doxycycline hyclate is [4S(4aR,5S,5aR,6R,12aS)]4(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-deoxonaphthacene-2-carboxamide monohydrochloride, compound with ethyl alcohol (2:1), monohydrate. Doxycycline hyclate is a yellow crystalline powder soluble in water and in solutions of alkali hydroxides and carbonates. Doxycycline has a high degree of lipid solubility and a low affinity for calcium binding. It is highly stable in normal human serum. Doxycycline will not degrade into an epianhydro form.
Each tablet contains doxycycline 120 mg (equivalent to doxycycline hyclate 138.8 mg). Inactive ingredients in the tablet formulation are: lactose monohydrate; microcrystalline cellulose; sodium lauryl sulfate; sodium chloride; talc; anhydrous lactose; corn starch; crospovidone; magnesium stearate; cellulosic polymer coating.
Each DORYX MPC 120 mg Tablet contains 7.2 mg (0.313 mEq) of sodium.
| Dosage Forms and Strengths |
|---|
|
DORYX MPC (doxycycline hyclate delayed-release tablets), 120 mg are white, oval tablets containing yellow pellets and debossed on one face with “DC” and plain on the other. Each tablet contains doxycycline 120 mg (equivalent to doxycycline hyclate 138.8 mg). |
| How Supplied |
|---|
|
DORYX MPC (doxycycline hyclate delayed-release tablets), 120 mg are white, oval tablets containing yellow pellets and debossed on one face with “DC” and plain on the other. Each tablet contains doxycycline 120 mg (equivalent to doxycycline hyclate 138.8 mg). The 120 mg tablet is supplied in bottles of 30 tablets. NDC 51862-559-30 Manufactured by: Mayne Pharma International Pty Ltd, 1538 Main North Road, Salisbury South, SA 5106 Australia Distributed by: Mayne Pharma, Greenville, NC 27834 |
Drugs
| Drug | Countries | |
|---|---|---|
| DORYX | Australia, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.