DRAXIMAGE DTPA Kit, Powder for solution for injection Ref.[49664] Active ingredients: Technetium ⁹⁹ᵐTc pentetic acid
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
11.1 Chemical Characteristics
DRAXIMAGE DTPA is a kit for the preparation of Technetium Tc 99m pentetate injection, a radioactive diagnostic agent, for intravenous or inhalation use. Each multiple-dose 10 mL glass vial contains a sterile, non-pyrogenic, non-radioactive lyophilized powder of 20 mg of pentetic acid, 5 mg of p-aminobenzoic acid, 3.73 mg of calcium chloride dihydrate, and not less than 0.25 mg stannous chloride dihydrate and not more than 0.385 mg maximum tin expressed as stannous chloride dihydrate. The lyophilized product is sealed under an atmosphere of nitrogen. No bacteriostatic preservative is present. Its chemical name is:
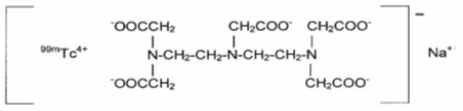
Technetate (1-)99mTc,[N,N-bis[2-[bis(carboxymethyl)amino]ethyl]glycinato(5)]-, sodium. The structure of the technetium labeled form is:
The pH is adjusted with HCl and/or NaOH prior to lyophilization so that the pH range of the reconstituted radiopharmaceutical is 6.5 to 7.5.
11.2 Physical Characteristics
Technetium Tc 99m decays by isomeric transition with a physical half-life of 6 hours. The principal photon that is useful for detection and imaging studies is listed in Table 7.
Table 7. Principal Radiation Emission Data:
| Radiation | Mean % per Disintegration | Mean Energy (keV) |
|---|---|---|
| Gamma-2 | 88.5 | 140.5 |
The air-kerma-rate (exposure-rate) constant for Technetium Tc 99m is 5.23 m2·pGy·(MBq) -1·s-1 [0.795 cm2·R·(mCi) -1·h-1]. A range of values for the relative radiation attenuation by the various thicknesses of lead is shown in Table 8. For example, the use of a 3 mm thickness of lead will attenuate the radiation emitted by a factor of about 1,000.
Table 8. Radiation Attenuation by Lead Shielding:
| Shield Thickness (Pb) cm | Coefficient of Attenuation |
|---|---|
| 0.25 | 0.5 |
| 1 | 10-1 |
| 2 | 10-2 |
| 3 | 10-3 |
| 4 | 10-4 |
To correct for physical decay of this radionuclide, the fractions that remain at selected intervals after the time of calibration are shown in Table 9.
Table 9. Physical Decay Chart of Technetium 99mTc, Half Life: 6 Hours:
| Hours | Fraction Remaining | Hours | Fraction Remaining |
|---|---|---|---|
| 0* | 1.000 | 5 | 0.562 |
| 1 | 0.891 | 6 | 0.501 |
| 2 | 0.794 | 8 | 0.398 |
| 3 | 0.708 | 10 | 0.316 |
| 4 | 0.631 | 12 | 0.251 |
* Calibration Time
| Dosage Forms and Strengths |
|---|
|
Kit for the preparation of Technetium Tc 99m pentetate injection: multiple-dose 10 mL glass vial contains a non-radioactive (white) lyophilized powder with 20 mg of pentetic acid, 5 mg of p-aminobenzoic acid, 3.73 mg of calcium chloride dihydrate, and not less than 0.25 mg stannous chloride dihydrate and not more than 0.385 mg maximum tin expressed as stannous chloride dihydrate. The lyophilized product is sealed under an atmosphere of nitrogen. Following reconstitution with the Technetium Tc 99m eluate, the radioactive solution produced is a clear solution not exceeding 9250 MBq/mL (250 mCi/mL) of Tc 99m. |
| How Supplied |
|---|
|
DRAXIMAGE DTPA is supplied as multiple dose kits consisting of 10 mL reaction vials containing a white, lyophilized powder with 20 mg of pentetic acid, 5 mg of p-aminobenzoic acid, 3.73 mg of calcium chloride dihydrate, and not less than 0.25 mg stannous chloride dihydrate and not more than 0.385 mg tin expressed as stannous chloride dihydrate. The radionuclide is not part of the kit. Before reconstitution and radiolabeling with sodium pertechnetate Tc 99m injection USP, the contents of the kit are not radioactive. The kits are supplied in the following formats: Carton containing 5 (five) kits NDC 65174.288.05 |
Drugs
| Drug | Countries | |
|---|---|---|
| DRAXIMAGE DTPA | Hong Kong, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.